Spinal Muscular Atrophy Market Size
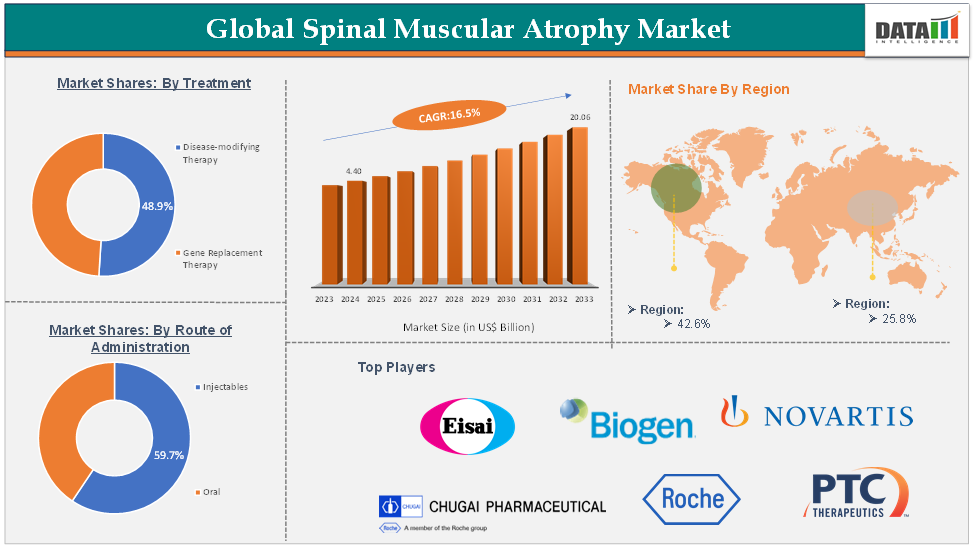
Spinal Muscular Atrophy Market size reached US$ 4.40 Billion in 2024 and is expected to reach US$ 20.06 Billion by 2033, growing at a CAGR of 16.5% during the forecast period 2025-2033.
The global market for spinal muscular atrophy (SMA) is poised for significant growth, driven by advancements in treatment options, especially the emergence of innovative gene therapies and ongoing clinical research. As of 2023, regulatory bodies, particularly in North America, have granted approvals for several of these therapies, further boosting market potential. The market's future growth is also supported by increasing awareness, expanding treatment accessibility, and robust investment in research and development.
Executive Summary
For more details on this report – Request for Sample
Spinal Muscular Atrophy Market Dynamics: Drivers & Restraints
Rising Approvals of Innovative Treatment Options is Expected to Drive the Spinal Muscular Atrophy Market
The rising approvals of innovative treatment options are playing a critical role in driving the Spinal Muscular Atrophy (SMA) market, particularly as new therapies expand access and improve outcomes for patients across all age groups. SMA, a rare genetic neuromuscular disorder affecting approximately 1 in 10,000 live births, is the leading genetic cause of infant mortality.
For instance, in May 2022, Roche announced that the U.S. Food and Drug Administration (FDA) approved a label extension for Evrysdi (risdiplam) to include babies under two months old with spinal muscular atrophy (SMA). This approval was based on promising interim efficacy and safety data from the RAINBOWFISH study, which demonstrated that the majority of pre-symptomatic infants treated with Evrysdi achieved key developmental milestones.
After 12 months of treatment, these infants were able to sit, stand, and even walk, highlighting the potential of early intervention in improving outcomes for SMA patients. The approval also incorporated two-year pooled data from the FIREFISH study, which demonstrated that a significant proportion of symptomatic infants treated with Evrysdi were able to sit or stand independently.
These advancements not only provide broader treatment access but also highlight the growing confidence in early, gene-targeting interventions, thereby fueling ongoing R&D investments and significantly boosting the SMA market.
High Cost of Treatment is Expected to Hinder the Spinal Muscular Atrophy Market
The cost of treatment for Spinal Muscular Atrophy (SMA) is extremely high, posing a significant barrier to market growth and patient access. Zolgensma, a one-time gene therapy developed by Novartis, is priced at over $2.1 million, making it one of the most expensive drugs globally. These high prices, even in countries with advanced healthcare systems, create major challenges for insurance coverage and reimbursement, limiting widespread adoption and accessibility.
Spinal Muscular Atrophy Market Segment Analysis
The global spinal muscular atrophy market is segmented based on type, treatment, route of administration, and region.
Treatment:
The gene replacement therapy segment is expected to hold 49.2% of the global spinal muscular atrophy market
The gene replacement therapy segment is projected to dominate the spinal muscular atrophy (SMA) market, driven by recent advancements and approvals that expand treatment accessibility and efficacy. In March 2023, the National Institute for Health and Care Excellence (NICE) in the UK recommended Zolgensma (onasemnogene abeparvovec) for routine use in infants with presymptomatic 5q SMA, marking a significant milestone in early intervention strategies.
This recommendation followed positive long-term data presented at the Muscular Dystrophy Association (MDA) Clinical & Scientific Conference in March 2023, where studies demonstrated that children treated with Zolgensma maintained and even gained motor milestones years after treatment.
Furthermore, in January 2025, Novartis announced that its intrathecal formulation of onasemnogene abeparvovec achieved the primary endpoint in a Phase III study involving pediatric patients aged 2–17 years with type II SMA, potentially broadening the eligible patient population for this gene transfer therapy. These developments underscore the transformative impact of gene replacement therapies in the SMA treatment landscape, contributing to their expected dominance in the market.
Spinal Muscular Atrophy Market Geographical Share
North America is expected to hold 42.6% of the global spinal muscular atrophy market
North America is set to continue its dominant role in the global spinal muscular atrophy (SMA) market, thanks to its advanced healthcare infrastructure, fast-paced drug approval processes, and significant investments in research and development.
The U.S., in particular, is leading the charge in the SMA landscape, largely due to regulatory bodies like the FDA, which have expedited the approval of groundbreaking therapies, including gene treatments like Zolgensma. This therapy has changed the way SMA is treated by addressing the genetic root cause of the disease, offering a chance for early intervention that dramatically improves patient outcomes.
For instance, in 2025, the FDA’s approval of a new tablet version of Evrysdi (risdiplam) from Genentech expanded treatment options for SMA patients of all ages. This approval reflects not only the increasing sophistication of SMA treatments but also the dedication to meeting the diverse needs of patients, whether through gene therapy or oral medications.
Furthermore, partnerships with research institutions and other pharmaceutical companies by the established players in the region are also contributing to the region’s market growth. These collaborations underscore North America's leadership in clinical trials, where innovative therapies are being rigorously tested and refined, providing patients access to some of the most advanced treatments available today.
With strong regulatory backing and ongoing innovation, North America is expected to remain at the forefront of SMA treatment development, maintaining its position as a key player in the global market in the future.
Asia-Pacific is expected to hold 25.8% of the global spinal muscular atrophy market
The Asia-Pacific region is expected to continue as the fastest-growing region in the spinal muscular atrophy market. This growth is fueled by the increasing incidence of the condition, rising research, and developmental activities in the region.
The expanding availability of advanced therapies such as Spinraza, Zolgensma, and Evrysdi is also fueling market growth. Japan plays a key role in this regional expansion, forecasted to grow steadily. This growth is supported by Japan’s advanced healthcare system, strong research initiatives targeting rare diseases, and high adoption rates of innovative gene therapies, positioning the country as a leader in SMA treatment within the Asia-Pacific landscape.
Spinal Muscular Atrophy Market Competitive Landscape
The top companies in the spinal muscular atrophy market include F. Hoffmann-La Roche Ltd, Novartis AG, Biogen, Chugai Pharmaceutical Co., Ltd., PTC Therapeutics, Inc., and Eisai Co., Ltd., among others.
Spinal Muscular Atrophy Market Key Developments
- In March 2025, Chugai Pharmaceutical Co., Ltd. announced that it received regulatory approval from Japan’s Ministry of Health, Labour and Welfare for the Evrysdi 5mg Tablets, a new oral formulation for spinal muscular atrophy (SMA) treatment. This tablet provides an additional option for SMA patients aged 2 years and older, weighing 20 kg or more, complementing the existing dry syrup formulation. Evrysdi remains the only orally administered SMA treatment.
- In September 2024, Biogen Inc. announced positive topline results from Part B of its Phase 2/3 DEVOTE study, showing that a higher-dose regimen of nusinersen significantly improved motor function in treatment-naïve infants with spinal muscular atrophy (SMA). The modified regimen, involving two 50 mg loading doses and 28 mg maintenance doses every four months, outperformed a matched untreated control group from the ENDEAR study, meeting the primary endpoint at six months.
Spinal Muscular Atrophy Market Scope
| Metrics | Details | |
| CAGR | 16.5% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Type | SMA type 0 (Congenital SMA), SMA type 1 (Severe SMA), SMA type 2 (Intermediate SMA), SMA type 3 (Mild), SMA type 4 (Adult) |
| Treatment | Disease-modifying Therapy, Gene Replacement Therapy | |
| Route of Administration | Injectables, Oral | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials, product pipelines, and forecasts upcoming advancements in medical devices and pharmaceuticals.
- Product Performance & Market Positioning: Analyze product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: Covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyzes competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient product delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global spinal muscular atrophy market report delivers a detailed analysis with 57 key tables, more than 46 visually impactful figures, and 168 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2024
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.