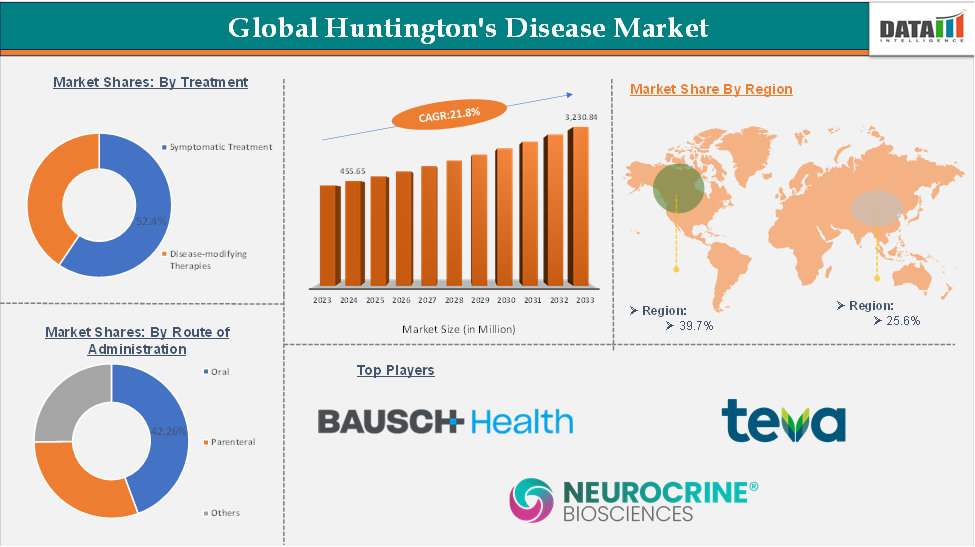
Huntington’s Disease Market Size
Huntington’s Disease Market size reached US$ 455.65 million in 2024 and is expected to reach US$ 3,230.84 million by 2033, growing at a CAGR of 7.5% during the forecast period 2025-2033.
The Huntington’s disease market is being driven by increasing research and development efforts focused on innovative therapies, alongside growing public awareness and education about the disorder. North America is expected to maintain a leading position in the global market due to strong healthcare infrastructure and ongoing clinical advancements, while the Asia-Pacific region is projected to experience the fastest growth, supported by expanding healthcare access and rising investment in neurological research.
Executive Summary
For more details on this report – Request for Sample
Huntington’s Disease Market Dynamics - Drivers & Restraints
Rising Research and Development is Expected to Propel the Huntington’s Disease Market
With increased focus on the genetic basis of the disease, therapies such as gene silencing and antisense oligonucleotides (ASOs), pharmaceutical companies, and research institutions continue to advance innovative therapeutic strategies. For instance, in June 2024, Wave Life Sciences announced encouraging results from its Phase 1b/2a SELECT-HD trial of WVE-003, an allele-selective ASO designed to reduce mutant huntingtin (mHTT) protein while preserving healthy wild-type HTT. The therapy showed a 46% reduction in mHTT in cerebrospinal fluid compared to placebo and demonstrated a significant correlation with slower brain atrophy, a biomarker linked to disease progression.
Additionally, organizations continue to invest heavily in HD research, accelerating the development of disease-modifying treatments. For instance, in January 2025, Brain Canada, the Huntington Society of Canada (HSC), and the River Philip Foundation jointly announced a new investment of $1.2 million to support research focused on Huntington’s disease. These advancements are expanding the therapeutic landscape and attracting regulatory interest, positioning R&D as a key force driving future growth in the Huntington’s disease market.
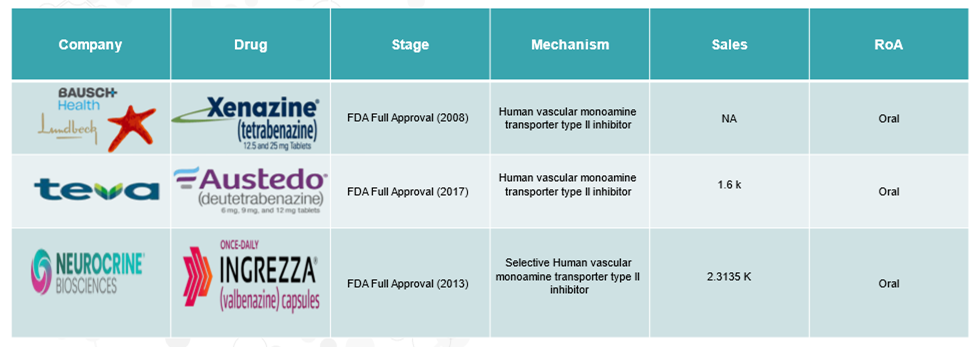
Huntington’s Disease Approved Drugs:
High Cost of Treatment is Expected to Hamper the Huntington’s Disease Market
The high cost of treatment could limit patient access and place a substantial financial burden on healthcare systems. Currently available therapies, such as Austedo (deutetrabenazine) and Xenazine (tetrabenazine), which are used to manage symptoms like chorea, can cost tens of thousands of dollars annually. These factors are expected to have a significant impact on the overall market growth.
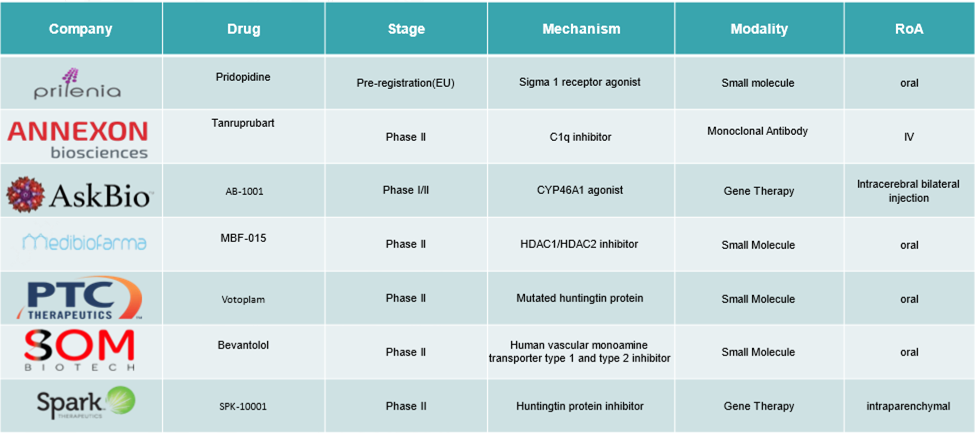
Huntington’s Disease Market - Pipeline Analysis
Huntington’s Disease Market Segment Analysis
The global Huntington’s disease market is segmented based on disease type, treatment, route of administration, and region.
Treatment:
The symptomatic treatment segment is expected to hold 52.4% of the global Huntington’s disease market
The symptomatic treatment segment is expected to dominate the Huntington’s disease market due to the well-established role of medications in managing hallmark symptoms, particularly chorea. Drugs such as Xenazine (tetrabenazine) and Austedo (deutetrabenazine) remain first-line therapies, offering substantial improvements in quality of life by reducing involuntary movements.
For instance, in Q2 2024, Teva Pharmaceutical Industries reported a 32% increase in quarterly U.S. sales of Austedo, reaching $407 million, highlighting its strong market demand and clinical relevance. Moreover, for instance, in August 2023, the FDA approved INGREZZA (valbenazine), developed by Neurocrine Biosciences, for the treatment of chorea associated with Huntington’s disease, further reinforcing the strength and expansion of symptomatic treatment options.
This dominance is further supported by disease prevalence data. For instance, according to the National Institute of Health in 2022, the diagnosed prevalence was estimated to be 8.2 to 9.0 per 100,000 in the United States. The growing pipeline of symptomatic therapies and their proven effectiveness make this segment likely to retain the largest market share as demand for symptom relief continues to rise.
Global Huntington’s Disease Market - Geographical Analysis
North America is expected to hold 39.7% of the global Huntington’s disease market
North America is expected to maintain a dominant position in the global Huntington’s disease (HD) treatment market due to its advanced healthcare infrastructure, high disease prevalence, and continuous research advancements. In recent years, significant developments in the region have further solidified this position. The U.S. Food and Drug Administration (FDA) has approved several treatments for symptomatic management of HD, including Austedo and Xenazine.
More recently, in August 2023, the FDA approved INGREZZA (valbenazine) for treating chorea associated with HD, based on positive results from the KINECT-HD Phase 3 study.
Additionally, companies are strategically partnering with each other to become prominent players in the market. For instance, UniQure reached an agreement with the FDA in December 2024 for the potential accelerated approval of its gene therapy AMT-130, aimed at addressing the root cause of HD.
Moreover, in December 2024, PTC Therapeutics partnered with Novartis in a licensing agreement valued at up to $2.9 billion for the development of PTC518, a drug targeting the mutated Huntingtin protein.
These efforts, along with proactive organizations like the Huntington Society of Canada, which works to train neurologists and improve care, position North America as a leader in advancing the treatment landscape for Huntington's disease.
Asia-Pacific is expected to hold 25.6% of the global Huntington’s disease market
The Asia-Pacific is expected to be the fastest-growing region in the Huntington’s disease market. This growth is attributed to the rising disease awareness, improved healthcare infrastructure, and increasing funding in research and therapeutic trials are major contributions.
Huntington’s Disease Market - Competitive Landscape
Teva Pharmaceutical Industries Ltd., Bausch Health Companies Inc., and Neurocrine Biosciences, Inc., among others.
Key Developments
- In September 2023, Ionis Pharmaceuticals, Inc. announced a collaboration with Roche focused on an early-stage RNA-targeting investigational therapy for Huntington’s disease. Under the agreement, Roche will have exclusive global rights and will lead clinical development, manufacturing, and commercialization if the treatment is approved. The partnership combines Ionis’ RNA-based drug discovery expertise with Roche’s global capabilities in neurological therapeutics.
Market Scope
| Metrics | Details | |
| CAGR | 21.8% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Mn) | |
| Segments Covered | Disease Type | Adult Onset, Early Onset |
| Treatment | Symptomatic Treatment, Disease-modifying Therapies | |
| Route of Administration | Oral, Parenteral, Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials, product pipelines, and forecasts upcoming advancements in medical devices and pharmaceuticals.
- Product Performance & Market Positioning: Analyze product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: Covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyzes competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient product delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global Huntington’s disease market report delivers a detailed analysis with 57 key tables, more than 46 visually impactful figures, and 168 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2024
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.