Gene Therapy Market Size
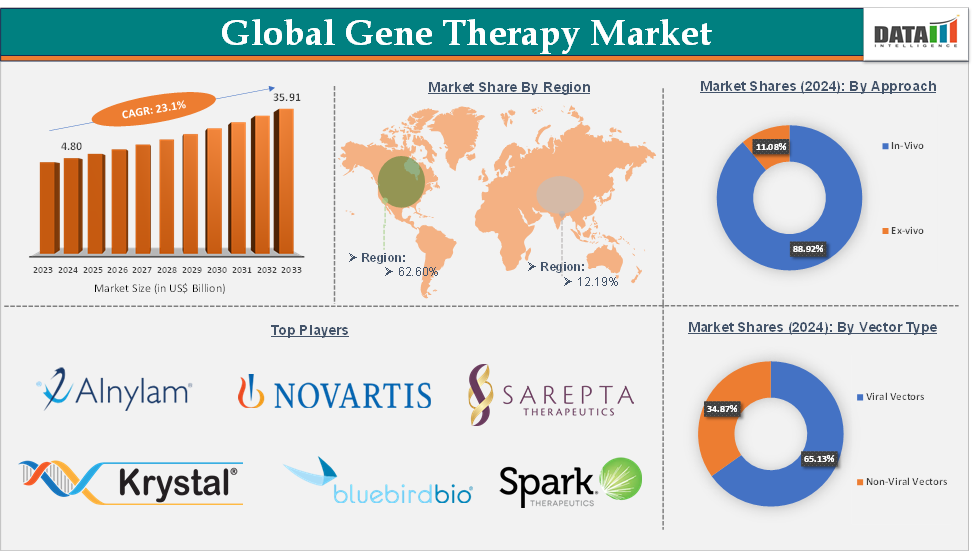
Gene Therapy Market size reached US$ 4.80 Billion in 2024 and is expected to reach US$ 35.91 Billion by 2033, growing at a CAGR of 23.1% during the forecast period 2025-2033.
Gene Therapy Market Overview
The gene therapy market is experiencing rapid growth, driven by advancements in genomics, biotechnology and personalized medicine. Gene therapy is revolutionizing genetic disorders, rare diseases and cancer treatments. The market has witnessed significant expansion due to the increasing R&D investments, strategic partnerships among key players and approval of novel gene therapies in emerging markets.
For instance, in January 2024, Vertex Pharmaceuticals Incorporated released the Saudi Food and Drug Authority (SFDA) granted Marketing Authorization for CASGEVY (exagamglogene autotemcel [exa-cel]), a CRISPR/Cas9 gene-edited therapy, for the treatment of sickle cell disease (SCD) and transfusion-dependent beta-thalassemia (TDT). CASGEVY is approved for treating people 12 years of age and older with SCD or TDT.
Additionally, gene therapy transforming oncology by developing products for various types of cancers. For instance, in January 2023, Ferring Pharmaceuticals cleared that ADSTILADRIN (nadofaragene firadenovec-vncg) is fully available across the U.S. for healthcare providers to prescribe for their adult patients with high-risk Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors. Approved by the U.S. Food & Drug Administration (FDA), ADSTILADRIN is the first and only FDA-approved intravesical gene therapy for adults with NMIBC who no longer respond to standard therapy.
Executive Summary
For more details on this report – Request for Sample
Gene Therapy Market Dynamics: Drivers & Restraints
Expansion into the rare diseases is significantly driving the gene therapy market growth
Rare diseases, which are genetic in nature, often have limited or no effective treatment options, making them ideal targets for gene therapies. The unique nature of these diseases presents a substantial opportunity for gene therapy to provide curative treatments where traditional approaches fail. For instance, Luxturna, a gene therapy treating children and adult patients with an inherited form of vision loss that may result in blindness. Luxturna is the first directly administered gene therapy approved in the U.S. that targets a disease caused by mutations in a specific gene.
Spinal Muscular Atrophy (SMA) is a rare genetic disorder that leads to progressive muscle weakness. Zolgensma, a gene therapy, offers a one-time treatment that has demonstrated significant improvements in survival and motor function for children with SMA. Hemophilia, another rare genetic disorder, requires lifelong treatment with clotting factor concentrates.
For instance, in February 2025, CSL Behring announced the four-year results from the pivotal HOPE-B study confirming the long-term durability and safety of a one-time infusion of HEMGENIX (etranacogene dezaparvovec-drlb) gene therapy for adults living with hemophilia B. As more gene therapies for rare diseases gain approval, the market will see a diversification of therapies, addressing a wider range of conditions.
High cost associated with the gene therapies are hampering the market growth
The high cost of gene therapies is one of the most significant challenges impacting the growth and accessibility of the gene therapy market. While gene therapies have shown curative potential for many serious diseases, their expensive price tags pose barriers to widespread adoption. For instance, the gene therapies Zynteglo and Skysona are priced at US$ 2.8 million and US$ 3 million per dose. Moreover, Zolgensma has a reported list price of US$ 2.1 million.
The development of gene therapies is an expensive, lengthy process. Clinical trials for gene therapies often span several years and involve high patient monitoring and expensive regulatory approval procedures. For instance, Luxturna, a gene therapy for Leber’s congenital amaurosis, took over 10 years from development to approval, and its cost is $850,000 per patient.
Many gene therapies are personalized treatments that are tailored to the genetic makeup of individual patients, requiring bespoke production for each case. This individualization further increases costs. For instance, CAR-T therapies such as Yescarta and Kymriah are customized for each patient by collecting and modifying the patient’s own T-cells, a process that involves complex manufacturing and quality control measures. These therapies can cost over $300,000 to $400,000 per patient.
Gene Therapy Market, Segment Analysis
The global gene therapy market is segmented based on approach, vector type, technique, application, and region.
Vector Type:
The viral vectors segment is expected to hold 65.13% of the market share in 2024 in the gene therapy market
In 2022, the viral vectors segment represented one of the fastest-growing segments, reaching US$ 6.25 billion, and further increased to US$ 6.75 billion in 2023.
Viral vectors are modified viruses used as delivery vehicles to introduce genetic material into cells for gene therapy. They exploit viruses' natural ability to transport their genomes into host cells, a process called transduction. The choice of viral vector depends on factors such as efficiency, safety, toxicity, stability, and the desired duration of transgene expression.
Currently, there are four types of viral vectors used in gene therapy: adenovirus, adeno-associated virus (AAV), lentivirus, and gamma-retrovirus (γ-retroviral). Each vector type requires a complex manufacturing process. These vectors are chosen for their ability to efficiently deliver genetic material, each having its strengths and weaknesses depending on the specific therapeutic application.
Furthermore, key players’ strategies such as partnerships, collaborations, and technological advancements would propel this segment’s growth. For instance, in October 2024, Roche is partnering with Dyno Therapeutics to develop next-generation adeno-associated virus (AAV) vectors for gene therapies targeting neurological diseases, potentially worth over $1 billion to Dyno. This collaboration, the second between the companies, grants Roche further access to Dyno's platform and sequence design technologies for in-vivo gene delivery.
Also, in March 2024, Charles River Laboratories International, Inc. introduced Modular and Fast Track frameworks to streamline viral vector technology transfer to its Maryland-based Center of Excellence (CoE). This program, leveraging decades of viral vector contract development and manufacturing organization (CDMO) experience, aims to expedite process transfer in as little as nine months. These frameworks provide stability, prevent program delays, and offer a cohesive "concept-to-cure" solution for gene therapy developers. Hence, the above-mentioned factors help the viral vectors segment to grow during the forecast period.
Gene Therapy Market Geographical Share
North America is expected to dominate the global gene therapy market with 62.60% share in 2024
North America led the Global Gene Therapy Market in 2022 with a market size of US$ 4.19 billion and expanded further to US$ 4.52 billion in 2023.
The gene therapy market in the North America region is expected to be driven by various factors like the increasing prevalence of cancer, genetic disorders, and other rare diseases. As per clinical Case Reports research publication in February 2024, SMA, a genetic disorder resulting from mutations in both copies of the SMN1 gene in cells, affects 1 in 6,000 to 1 in 10,000 children. According to the National Heart, Lung, and Blood data in September 2024, Sickle cell disease (SCD) affects approximately 100,000 people in the United States, with over 90% being non-Hispanic Black or African American, and 8 million people worldwide. The demand for gene-based treatments is expected to accelerate, driving the overall growth of the gene therapy market.
Furthermore, in this region, a major number of key players’ presence, a well-advanced healthcare infrastructure, technological advances, product launches, approvals, and increasing research activities are driving the gene therapy market in the North American region.
For instance, in September 2024, Vertex Pharmaceuticals Incorporated announced that Health Canada granted marketing authorization for CASGEVY (exagamglogene autotemcel) for individuals 12 years and older with sickle cell disease (SCD) with recurrent vaso-occlusive crises (VOCs) or transfusion-dependent beta-thalassemia (TDT). CASGEVY is an autologous genome-edited hematopoietic stem cell-based therapy. It is estimated that approximately 2,000 patients in Canada are eligible for this treatment, with the majority having SCD.
Similarly, in March 2024, Orchard Therapeutics, acquired by Kyowa Kirin, announced its U.S. launch plans for Lenmeldy (atidarsagene autotemcel), the first FDA-approved gene therapy for children with early-onset metachromatic leukodystrophy (MLD). MLD is a rare, rapidly progressive, and ultimately fatal neurometabolic disease affecting roughly one in 100,000 live births.
Gene Therapy Market Top Companies
Top companies in the gene therapy market include Alnylam Pharmaceuticals, Inc., Spark Therapeutics, Inc., Novartis AG, bluebird bio, Inc., Ferring Pharmaceuticals Inc., Vertex Pharmaceuticals Incorporated, Sarepta Therapeutics, Inc., CSL Behring LLC, Amgen, Inc., Orchard Therapeutics group., Krystal Biotech, Inc., and among others.
Market Scope
| Metrics | Details | |
| CAGR | 23.1% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Approach | Ex-Vivo and In-Vivo |
| Vector Type | Viral Vectors and Non-Viral Vectors | |
| Technique | Gene Addition, Gene Silencing, and Gene Editing | |
| Application | Oncology, Musculoskeletal Conditions, Blood Disorders, Rare Diseases, Ophthalmology, and Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global gene therapy market report delivers a detailed analysis with 73 key tables, more than 64 visually impactful figures, and 168 pages of expert insights, providing a complete view of the market landscape.