Market Size
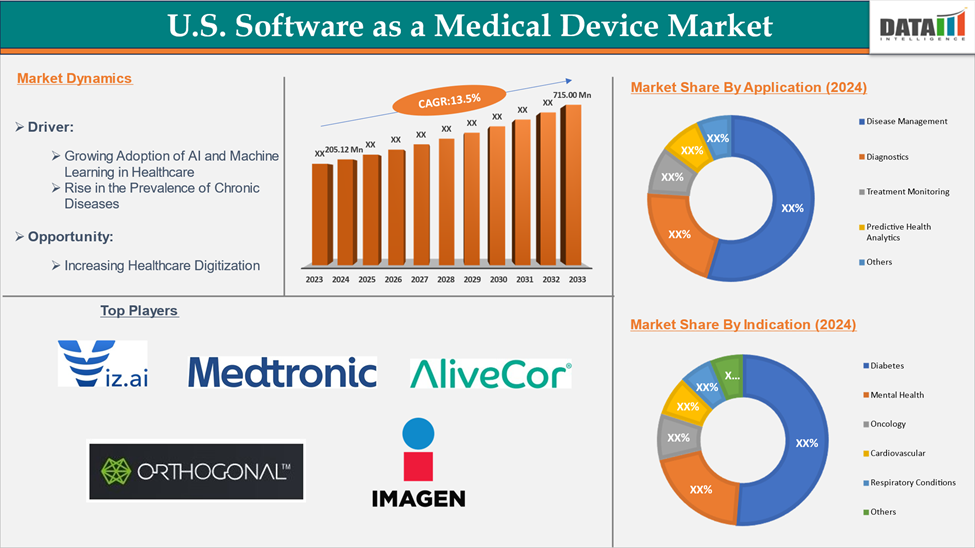
The US Software as a Medical Device Market reached US$ 205.12 million in 2024 and is expected to reach US$ 715.00 million by 2033, growing at a CAGR of 13.5% during the forecast period 2025-2033.
Software as a Medical Device (SaMD) is a standalone software that performs medical functions without being physically integrated into a traditional device. It diagnoses, prevents, monitors, or treats diseases by processing data, providing clinical insights, and assisting in decision-making.
SaMD functions independently on platforms like smartphones, cloud-based systems, or desktop applications. The increasing adoption of AI and cloud computing has expanded its applications in remote patient monitoring, diagnostics, and digital therapeutics.
Executive Summary
For more details on this report – Request for Sample
Market Dynamics: Drivers & Restraints
Improved Real-Time Monitoring and Predictive Analytics
The integration of AI and machine learning is revolutionizing the U.S. Software as a Medical Device (SaMD) market by improving diagnostic precision, automating clinical workflows, and enabling real-time patient monitoring. AI-powered SaMD solutions detect diseases early, personalize treatment plans, and enhance healthcare providers' decision-making. Regulatory bodies like the FDA are supporting AI-driven innovations, driving market growth and technological advancements in healthcare systems.
For instance, in January 2023, Viz.ai has launched VizTM Vascular Suite, an AI-powered software that enables vascular care teams to automatically detect and triage suspected pulmonary embolism, right heart strain, aortic dissection, and abdominal aortic aneurysm. The company has submitted a new application to the FDA for the AAA algorithm.
Regulatory and Compliance Challenges
The U.S. Software as a Medical Device (SaMD) market is shaped by regulatory approvals and compliance, with the FDA imposing rigorous evaluations to ensure safety, efficacy, and cybersecurity. These regulations can lead to extended product development cycles and increased costs for manufacturers. Compliance with data privacy laws like HIPAA complicates market entry, requiring robust security frameworks. The evolving regulatory guidelines for AI-driven SaMD add complexity, making it challenging for companies to innovate and scale their solutions.
Market Segment Analysis
The U.S. software as a medical device market is segmented based on application and indication.
Application:
Disease management from the application segment is expected to dominate the software as a medical device market with the highest market share
Disease management is a structured approach to improve health outcomes for chronic conditions through coordinated care, patient education, and data-driven interventions. It involves monitoring disease progression, adherence to treatment, and lifestyle modifications. Technology can optimize treatment plans, enhance patient compliance, and reduce hospitalizations.
Disease management is a key aspect of the U.S. Software as a Medical Device (SaMD) market, providing real-time data collection, remote patient monitoring, and decision support for chronic and acute conditions. These tools, which offer AI-driven insights and automated alerts, improve patient outcomes and reduce healthcare costs. The integration of disease management within SaMD enhances patient engagement, streamlines clinical workflows, and supports evidence-based care, driving its adoption in the U.S. healthcare landscape.
Major US Players
The major U.S players in the software as a medical device market include Viz.ai, Inc., Medtronic Plc, AliveCor, Empatica, Bigfoot Biomedical, Digital Diagnostics Inc., Imagen and Orthogonal among others.
Key Developments
- In January 2025, Caranx Medical, a medical device company, submitted the world's first AI Software for real-time intra-operative guidance of Transcatheter Aortic Valve Implantation (TAVI), a lifesaving procedure, to the FDA.
- In January 2024, Siemens Healthineers received FDA clearance for Syngo Virtual Cockpit, a secure communication platform for real-time image visualization and collaboration between healthcare professionals across multiple sites. This is the first multi-vendor remote scanning software as a medical product.
Market Scope
| Metrics | Details | |
| CAGR | 13.5% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Mn) | |
| Segments Covered | Application | Disease Management, Diagnostics, Treatment Monitoring Predictive Health Analytics, Others |
| Indication | Diabetes, Mental Health, Oncology, Cardiovascular, Respiratory Conditions, Others | |
Why Purchase the Report?
- Technological Innovations: Reviews ongoing clinical trials, product pipelines, and forecasts upcoming advancements in medical devices and pharmaceuticals.
- Product Performance & Market Positioning: Analyzes product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: Covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyzes competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient product delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The U.S software as a medical device market report delivers a detailed analysis with 30 key tables, more than 20 visually impactful figures and 159 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2024
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Application & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.