Global Turner Syndrome Market: Industry Outlook
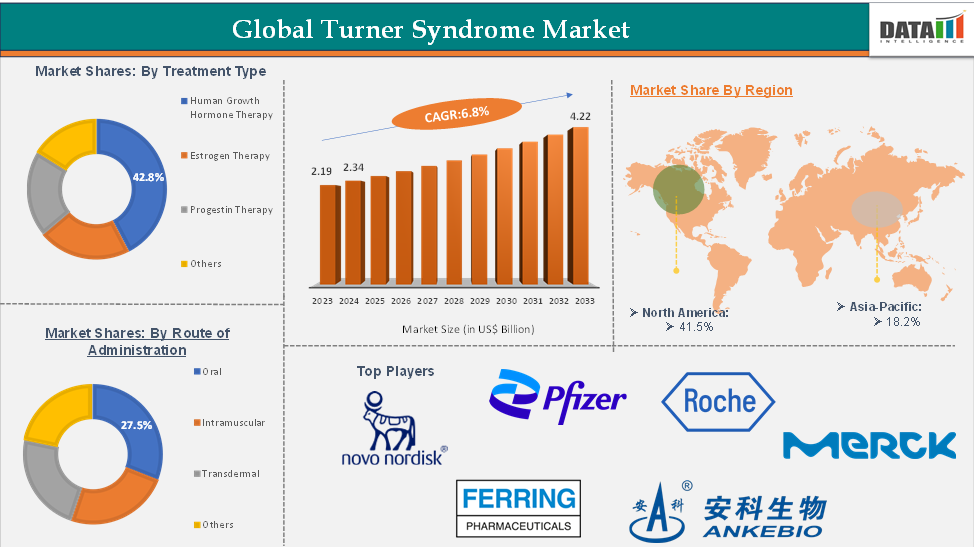
The global Turner syndrome market reached US$ 2.19 billion in 2023, with a rise of US$ 2.34 billion in 2024, and is expected to reach US$ 4.22 billion by 2033, growing at a CAGR of 6.8% during the forecast period 2025-2033.
The Turner syndrome market is steadily advancing, driven by growing awareness, earlier diagnosis, and improvements in hormone therapies. Growth hormone remains the foundation of care, with increasing use of long-acting options like TransCon hGH that reduce injection frequency and improve patient comfort. Estrogen replacement therapy also plays a critical role, particularly in supporting puberty and long-term bone and heart health, with newer delivery methods offering more flexibility and fewer side effects. Rising attention to pediatric genetic conditions is leading to earlier identification and better access to treatment, especially in specialized care settings.
Additionally, increased focus on female-specific genetic disorders is expanding recognition and support globally. Ongoing research and tailored treatment approaches are helping to reduce complications and improve outcomes, reinforcing Turner Syndrome as a key area of growth in pediatric and hormonal disorder management.
Global Turner Syndrome Market: Executive Summary
Global Turner Syndrome Market Dynamics: Drivers & Restraints
Driver: Increased Prevalence of Infectious Diseases and Chronic Conditions
Increased awareness and early diagnosis of Turner syndrome are expected to be key drivers of market growth over the coming years. With rising efforts from healthcare organizations, rare disease advocacy groups, and public health campaigns, there is greater recognition of TS symptoms among both medical professionals and the general public. For instance, according to a 2025 publication by the National Institutes of Health (NIH), Turner syndrome occurs in approximately 1 in every 2,000 to 2,500 live female births. Based on recent nationwide database estimates, the prevalence is approximately 3.2 per 10,000 female live births when considering all pregnancy outcomes. When limited to live births and stillbirths, the prevalence drops to around 1.9 per 10,000. Notably, the condition is significantly more common during the antenatal period, as a majority of affected pregnancies result in spontaneous miscarriage before birth. This has led to more frequent and earlier diagnosis, often in infancy or early childhood, thanks to expanded use of genetic testing and karyotyping.
As awareness grows, so does the number of diagnosed and treated cases, increasing demand for approved therapies like recombinant human growth hormone (e.g., Norditropin, Genotropin) and transdermal estradiol. Moreover, increased diagnosis also supports long-term monitoring and the use of adjunct therapies for associated complications, such as cardiovascular or renal anomalies, further expanding the market. Healthcare systems in developed and emerging markets alike are beginning to prioritize early TS screening as part of pediatric growth monitoring programs, creating a positive feedback loop that enhances both treatment uptake and commercial opportunities for pharmaceutical and biotech firms.
Restraint: High Cost of Therapy
The high cost of therapy remains a significant barrier that could hamper the growth of the Turner Syndrome (TS) treatment market. Management of TS typically requires long-term, multi-faceted care, regular medical evaluations, and monitoring for associated complications such as cardiac or renal issues. Growth hormone therapies, especially newer long-acting formulations like lonapegsomatropin (TransCon hGH), can be particularly expensive, often costing tens of thousands of dollars per year. Estrogen replacement, while relatively more affordable, still contributes to long-term healthcare costs.
Global Turner Syndrome Market Segment Analysis
The global Turner syndrome market is segmented based on type, treatment type, route of administration, end-user, and region.
Treatment Type:
The human growth hormone (HGH) therapy segment is estimated to have 42.8% of the Turner syndrome market share.
Human growth hormone (HGH) therapy is poised to dominate the Turner Syndrome (TS) treatment market due to its proven efficacy and recent advancements in treatment formulations. A notable development is the approval of TransCon hGH (lonapegsomatropin), a once-weekly long-acting growth hormone, for pediatric use in TS. In December 2024, Ascendis Pharma announced positive Week 26 topline results from the New InsiGHTS Phase 2 trial, demonstrating that TransCon hGH provided comparable safety and efficacy to daily somatropin injections in prepubertal children with TS.
The efficiency of TransCon hGH is driving market growth by offering improved adherence and convenience. Unlike daily injections, the once-weekly regimen reduces the treatment burden on patients and caregivers, potentially leading to better long-term outcomes. This advancement aligns with the growing preference for less invasive and more manageable treatment options in chronic conditions.
As the treatment landscape for Turner syndrome evolves, the introduction of long-acting HGH therapies like TransCon hGH is expected to play a pivotal role in enhancing patient outcomes and driving the growth of the TS treatment market.
Global Turner Syndrome Market Geographical Share
The North America turner syndrome market was valued at 41.5% market share in 2024
North America is expected to dominate the Turner Syndrome (TS) treatment market due to its advanced healthcare infrastructure, high awareness levels, and strong government support. The region, particularly the United States, benefits from widespread access to specialized medical care and extensive newborn screening programs, which facilitate early diagnosis and timely intervention.
Additionally, substantial funding for genetic and rare disease programs ensures better availability of diagnostic tools and treatments. Patients in North America also have access to cutting-edge therapies, including recombinant human growth hormone (rhGH) and innovative estrogen delivery systems, which improve patient outcomes and quality of life. For instance, in June 2023, Pfizer Inc. and OPKO Health Inc. announced that the U.S. Food and Drug Administration (FDA) approved NGENLA (somatrogon‑ghla), a novel long-acting human growth hormone analog administered once weekly, for the treatment of pediatric patients aged three years and older with growth failure due to inadequate endogenous growth hormone secretion. These combined factors position North America as the leading market for TS treatment, driving sustained growth and continued innovation in this field.
Global Turner Syndrome Market Major Players
The major players in the Turner syndrome market include Pfizer Inc., Novo Nordisk A/S, Eli Lilly and Company, Ferring, F. Hoffmann-La Roche Ltd, AnkeBio Co., Ltd, Merck KGaA, among others.
Global Turner Syndrome Market Scope
Metrics | Details | |
CAGR | 6.8% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Type | Classical Turner Syndrome, Mosaic Turner Syndrome |
Treatment Type | Human Growth Hormone Therapy, Estrogen Therapy, Progestin Therapy, Others | |
| Route of Administration | Oral, Intramuscular, Transdermal, Others |
| End-User | Hospitals, Specialty Clinics, Homecare, Others |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
For more details on this report, Request for Sample
The global Turner syndrome market report delivers a detailed analysis with 73 key tables, more than 76 visually impactful figures, and 195 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more pharmaceuticals-related reports, please click here