Genetic Testing Market Size
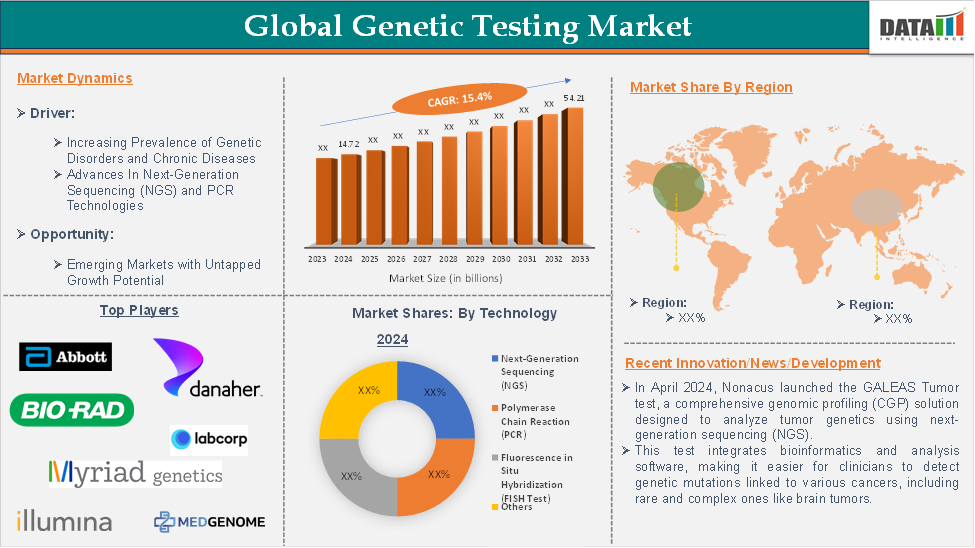
The global genetic testing market size reached US$14.72 billion in 2024 and is expected to reach US$54.21 billion by 2033, growing at a CAGR of 15.4 % during the forecast period of 2025-2033, according to DataM Intelligence report.
The global genetic testing market refers to the use of diagnostic tests that analyze DNA, RNA, or proteins to identify genetic conditions, hereditary diseases, and the risk of future health conditions. This market includes various types of genetic tests, such as carrier testing, diagnostic testing, predictive testing, prenatal testing, and pharmacogenomics. These tests help in diagnosing diseases, understanding genetic predispositions, and enabling personalized treatment strategies.
Genetic testing allows for personalized treatment strategies, tailoring healthcare to individual genetic profiles. It aids in early diagnosis, improving patient outcomes by identifying the best treatment options and avoiding adverse drug reactions.
Executive Summary
For more details on this report Request for Sample
Genetic Testing Market Dynamics: Drivers & Restraints
Increasing Prevalence of Genetic Disorders and Chronic Diseases
The increasing prevalence of genetic disorders and chronic diseases is expected to significantly drive the growth of the genetic testing market. As the incidence of genetic conditions like cystic fibrosis, sickle cell anemia, and Huntington’s disease continues to rise, the demand for genetic testing is also growing. For instance, according to the Cystic Fibrosis Foundation, approximately 1,000 new cases of CF are diagnosed each year. This increase in the number every year increases the demand for advanced testing procedures to diagnose the condition at an early stage.
Early detection through genetic testing can help healthcare providers offer personalized treatment plans, potentially improving outcomes and reducing healthcare costs in the long run. In addition, the rising prevalence of chronic diseases such as cancer, diabetes, and cardiovascular conditions is increasing the need for genetic testing even more.
A large number of individuals suffering from chronic diseases are prescribed or are showing interest in genetic testing to diagnose the disease accurately. For instance, according to the NORC at the University of Chicago in 2022, it is stated that 17 percent of Americans have actually undergone genetic testing, and 52 percent report interest in such a test. Thus, the above factors are expected to drive the market growth.
High Cost of Genetic Tests and Sequencing Technologies
The high cost of genetic tests and sequencing technologies is expected to be a significant barrier to the growth of the genetic testing market. Despite advancements in genetic testing, the expenses associated with sequencing technologies, such as next-generation sequencing (NGS), remain relatively high, making these tests unaffordable for a large portion of the population.
This high cost can limit access to genetic testing, particularly for underserved populations in both developed and emerging markets. For example, many individuals may be unable to afford tests for disease risk assessment, prenatal screening, or personalized medicine, which could prevent early diagnosis or personalized treatment plans that could significantly improve health outcomes.
As a result, the high cost of genetic tests and sequencing technologies is likely to hinder the market's growth by limiting accessibility and reducing demand. Thus, the above factors could be limiting the global genetic testing market's potential growth.
Genetic Testing Market Segment Analysis
The global genetic testing market is segmented based on product type, technology, testing type, application, and region.
Technology:
The next-generation sequencing (NGS) segment in technology is expected to dominate the global genetic testing market with the highest market share
Next-Generation Sequencing (NGS) is a transformative technology in the genetic testing market, enabling the rapid and cost-effective sequencing of DNA and RNA. It is widely used in various applications, including clinical diagnostics, oncology, precision medicine, biomarker discovery, and agriculture. The global NGS market is experiencing strong growth, driven by advancements in sequencing technology, decreasing costs, and increasing demand for personalized medicine and genetic research.
For instance, in February 2025, Devyser launched Devyser Thalassemia v2, a next-generation sequencing (NGS) solution aimed at improving the accuracy and efficiency of genetic testing for alpha and beta thalassemia. This new version enhances the detection of structural variants and copy number variations (CNVs), providing broader and more precise screening capabilities. This innovation aligns with the growing demand for high-accuracy genetic diagnostics, particularly in the expanding NGS segment in the market.
Genetic Testing Market Geographical Analysis
North America is expected to hold a significant position in the global genetic testing market, with the highest market share
North America is expected to dominate the genetic testing market due to various significant factors. The growing prevalence of chronic diseases and genetic disorders in North America fuels the demand for genetic testing. As more individuals are diagnosed with conditions such as diabetes, cancer, and hereditary diseases, there is a growing demand for genetic testing to help in early diagnosis, risk assessment, and personalized treatment planning.
For instance, according to the report by the American Cancer Society in 2025, it is stated that approximately 1 in 8 women in the United States are diagnosed with breast cancer. Being one of the common cancers among women, the need for genetic testing is expected to increase, which is expected to drive the genetic testing market. Genetic testing detects inherited genetic mutations for various cancers.
Companies in the region are mainly focusing on developing advanced solutions for the diagnosis of genetic diseases to help in identifying the mutations in the genes and help in developing personalized medicine. For instance, in March 2024, Nucleus Genomics, a next-generation genetic testing and analysis company, launched its DNA analysis product aimed at making the benefits of personalized medicine accessible to everyone. This product is designed to empower individuals with the knowledge of their genetic makeup, helping them make informed decisions about their health.
Moreover, North America has one of the highest adoption rates of personalized medicine, where genetic tests play a crucial role in tailoring treatments to individual patients based on their genetic makeup. Thus, various factors are contributing to the region’s dominant position in the global genetic testing market.
Genetic Testing Market Major Players
The major global players in the genetic testing market include Abbott Laboratories, Bio-Rad Laboratories Inc., Illumina Inc., Myriad Genetics Inc., Danaher Corporation, Quest Diagnostics Incorporated, MedGenome, LabCorp, 3billion, Inc., and Blueprint Genetics, among others.
Key Developments
- In August 2024, Nonacus launched the GALEAS Tumor test, a comprehensive genomic profiling (CGP) solution designed to analyze tumor genetics using next-generation sequencing (NGS). This test integrates bioinformatics and analysis software, making it easier for clinicians to detect genetic mutations linked to various cancers, including rare and complex ones like brain tumors.
| Metrics | Details | |
| CAGR | 15.4% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Product Type | Consumables, Equipment, Software |
| Technology | Next-Generation Sequencing (NGS), Polymerase Chain Reaction (PCR), Fluorescence in Situ Hybridization (FISH Test), Others | |
| Testing Type | Carrier Testing, Prenatal and Newborn Testing, Preimplantation Testing, Predictive and presymptomatic testing, Forensic Testing, Diagnostic Testing, Others | |
| Application | Cancer Diagnosis, Genetic Disease Diagnosis, Cardiovascular Disease Diagnosis, Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials and product pipelines and forecasts upcoming advancements in medical devices and pharmaceuticals.
- Product Performance & Market Positioning: Analyzed product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: This covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyze competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient product delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global genetic testing market report delivers a detailed analysis with 70 key tables, more than 68 visually impactful figures, and 176 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2024
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.