Skin Allergy Testing Market Size& Industry Outlook
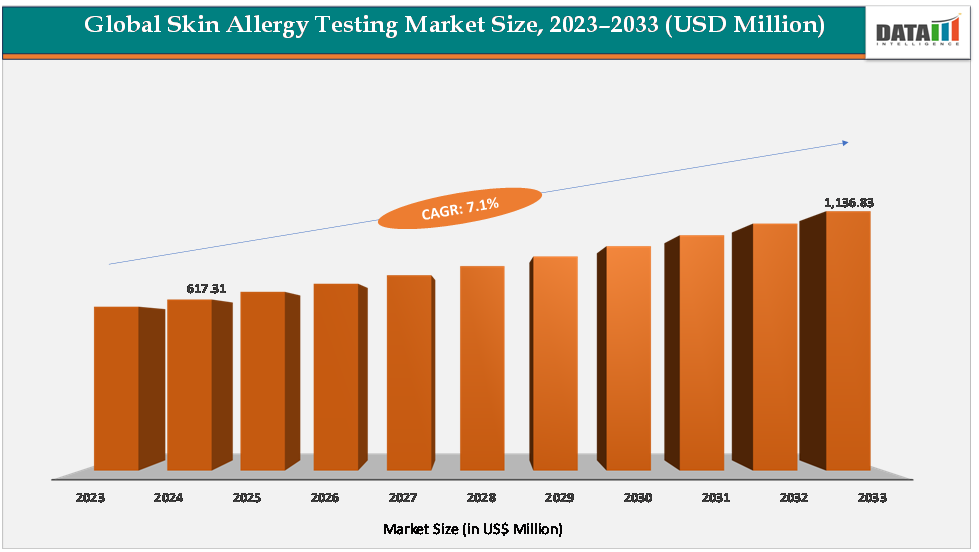
The global skin allergy testing market size reached US$617.31Million in 2024 from US$579.32Millionin 2023 and is expected to reach US$ 1,136.83Million by 2033, growing at a CAGR of 7.1%during the forecast period 2025-2033.
The market is growing steadily due to rising allergy prevalence worldwide, driven by urbanization, pollution, and changing lifestyles. Clinicians are increasingly relying on skin prick, intradermal, and patch tests as first-line diagnostic tools because they are cost-effective, rapid, and suitable for outpatient settings. At the same time, safety concerns and demand for more precise diagnosis are pushing adoption of in-vitro IgE tests and multiplex assays from leaders such as Thermos Fisher (ImmunoCAP) and HYCOR Biomedical. This dual demand for quick in-clinic tests plus advanced lab diagnostics is propelling the market’s expansion.
Key Market Highlights
North America dominates the skin allergy Testing Market with the largest revenue share of 43.17% in 2024.
The Asia Pacific is the fastest-growing region and is expected to grow at the fastest CAGR of7.3% over the forecast period.
Based on test type, the prick test segment led the market with the largest revenue share of 42.81% in 2024.
The major market players in the skin allergy testing market are Thermo Fisher Scientific Inc., Staller genes Greer, Siemens Healthiness, Allergy Therapeutics, Chemo technique MB Diagnostics AB, HAL Allergy B.V., Inmunotek, and HYCOR Biomedical, among others
Market Dynamics
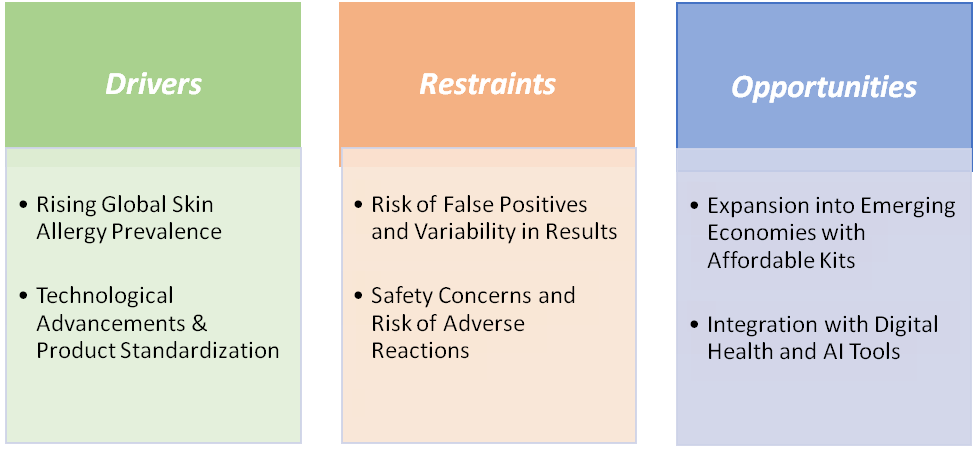
Drivers: Rising global skin allergy prevalence is significantly driving the skin allergy testing market growth
The rising global prevalence of skin allergies has become one of the most powerful forces driving the growth of the skin allergy testing market. Today, more people than ever are experiencing allergic conditions such as eczema, contact dermatitis, urticaria, and food-triggered skin reactions, fueled by factors like increasing urban pollution, climate change, dietary shifts, and heightened exposure to chemicals in cosmetics and personal care products. This surge in cases naturally pushes patients and clinicians to seek faster, more reliable diagnoses.
Dermatologists rely heavily on patch tests to identify triggers in patients suffering from chronic contact dermatitis caused by everyday exposures to fragrances, metals, or hair dyes. Similarly, allergists use the skin prick test (SPT) as the frontline tool to screen for common respiratory and food allergens, with standardized extracts supplied by companies such as Staller genes Greer. In hospital allergy clinics, the demand for intradermal tests is also increasing for more precise drug and insect venom allergy detection. The sharp rise in pediatric allergies has made these tools even more indispensable, since they offer quick, safe, and cost-effective results, helping clinicians reassure families while guiding treatment plans.
Restraints: Risk of false positives and variability in results are hampering the growth of the market
One of the biggest restraints in the skin allergy testing market is the risk of false positives and variability in results, which directly affects physician confidence and patient trust. Skin prick and intradermal tests, while widely used, can produce false positives due to cross-reactivity between similar allergens. Even the subjectivity of manual interpretation by different clinicians adds variability, since the measurement of redness or swelling is often done visually without standardized tools. This inconsistency sometimes forces physicians to order confirmatory in-vitro IgE assays like Thermos Fisher’s ImmunoCAP, increasing costs and delaying diagnosis.
In chronic conditions such as eczema or urticaria, where baseline skin reactivity is already high, false positives can complicate patient management and erode trust in skin tests. Over time, such issues push healthcare providers to prefer lab-based molecular assays, which are perceived as more reliable, thereby hampering the growth of traditional skin testing methods despite their cost-effectiveness and accessibility.
For more details on this report – Request for Sample
Segmentation Analysis
The global skin allergy testing market is segmented based on test type, end-user, and region.
Test Type:
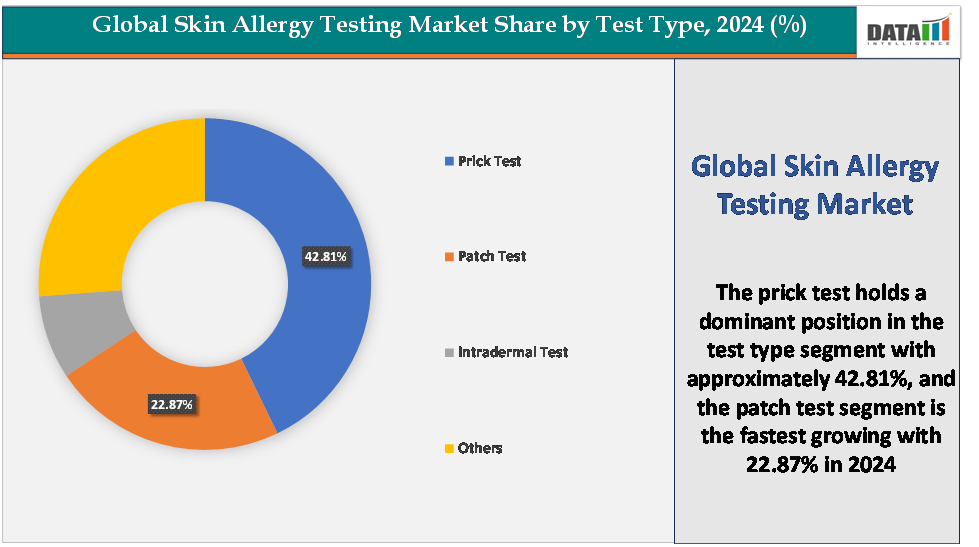
The prick test segment is dominating the skin allergy testing market with a 42.81% share in 2024
The skin prick test segment holds a dominant position in the skin allergy testing market, and its leadership is driven by a mix of clinical reliability, safety, and broad applicability. The skin prick test is universally recognized as the first-line diagnostic tool for IgE-mediated allergies, especially respiratory and food allergies, because it is quick, minimally invasive, cost-effective, and provides results within 15–20 minutes. Unlike intradermal tests, which carry a higher risk of systemic reactions, skin prick test offers a balanced profile of safety and diagnostic value, making it the go-to choice in routine allergy practice. Its dominance is further reinforced by the wide availability of standardized allergen extracts and testing kits approved by regulatory bodies.
For instance, Staller genes Greer markets FDA-cleared products like Skintestor OMNI and the GREER Pick applicator, both popular in the U.S. market. These standardized reagents ensure reproducibility, which strengthens clinician confidence. Recent guidelines by allergy associations also reinforce the prick test as a recommended first diagnostic step, which guarantees its ongoing central role despite the growth of molecular diagnostics. Together, these clinical advantages and real-world usage trends explain why the prick test segment remains dominant, serving as the backbone of allergy diagnostics worldwide.
The patch test segment is the fastest-growing segment in the skin allergy testing market, with a 22.87% share in 2024
The patch test segment is emerging as the fastest-growing area within the skin allergy testing market, fueled by rising awareness of contact dermatitis and increasing exposure to chemicals, metals, cosmetics, and occupational allergens. Their growth is being propelled by the surge in chronic skin conditions linked to lifestyle and occupational factors. Clinically, patch tests are gaining traction because they offer comprehensive allergen panels that screen for common culprits like nickel, cobalt, fragrance mix, and formaldehyde, which are often overlooked in routine allergy evaluations.
Regulatory support has also accelerated this growth. Several approved patch test systems are supporting this growth. The TRUE Test panels, FDA-approved and CE-marked, are widely used across North America and Europe to screen for standardized allergens, while extended allergen series from companies like Chemotechnique Diagnostics offer dermatologists region-specific allergen panels. The combination of expanding allergen panels, strong regulatory drivers, and increasing consumer awareness has positioned patch testing as the fastest-growing segment, even though its overall market share remains smaller than prick testing. In essence, the patch test market is riding the wave of lifestyle changes and occupational safety trends, ensuring rapid expansion in both developed and emerging regions.
Geographical Analysis
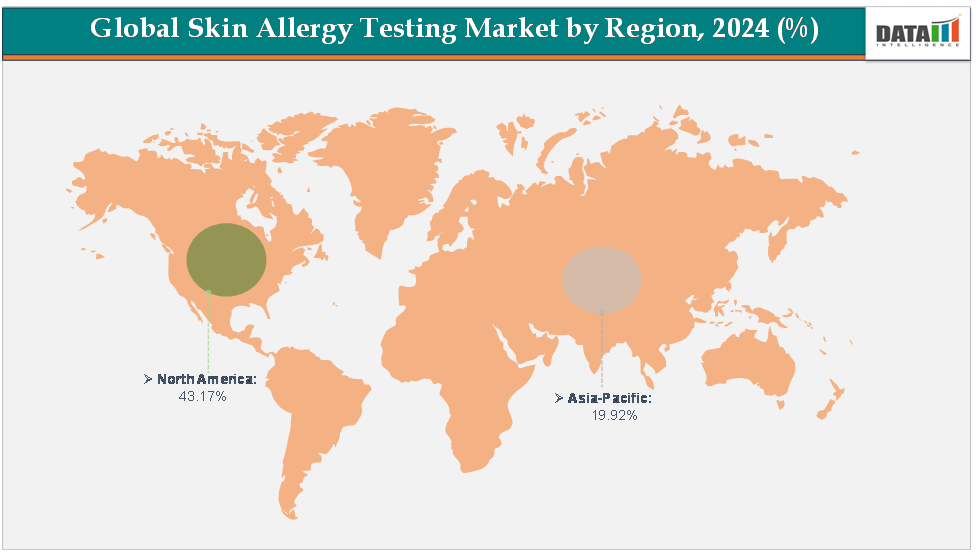
North America is expected to dominate the global skin allergy testing market with a 43.17% in 2024
North America stands as the dominant region in the global skin allergy testing market, and this leadership is rooted in a combination of high disease prevalence, advanced healthcare infrastructure, favorable reimbursement policies, and the presence of major market players.
US Skin Allergy Testing Market Trends
The US reports some of the highest allergy rates worldwide, with the Asthma and Allergy Foundation of America estimating that over 50 million Americans experience allergies annually, with skin conditions like atopic dermatitis, urticaria, and contact dermatitis being particularly common. This large patient pool naturally drives demand for first-line diagnostic tools, especially skin prick tests, which are widely used in hospitals, allergy clinics, and dermatology practices.
Companies such as Staller genes Greer play a pivotal role in this dominance, offering FDA-cleared products like the Skintestor OMNI kit and the GREER Pick applicator, which are staples in US allergy practices. In addition, the TRUE Test patch panels, also FDA-approved, are extensively adopted by dermatologists to diagnose contact dermatitis from cosmetics, fragrances, and metals, reflecting the region’s heightened awareness of lifestyle-driven skin allergies. The US also benefits from robust reimbursement frameworks through private insurers and Medicare/Medicaid, which cover allergy testing and make it accessible to broader patient groups.
Furthermore, the US advanced clinical guidelines from organizations such as the American Academy of Allergy, Asthma & Immunology (AAAAI) reinforce skin prick and patch testing as first-line diagnostics, ensuring consistent clinical adoption. The strong presence of global diagnostic leaders like Thermo Fisher Scientific (with its ImmunoCAP assays for confirmatory testing) also fosters an integrated ecosystem where skin tests are complemented by in-vitro solutions, further strengthening US dominance.
Regulatory support with the US FDA has also accelerated the market growth in the US. For instance, in February 2025, ALK announced that the U.S. Food and Drug Administration (FDA) expanded its indication of ODACTRA (House Dust Mite Allergen Tablet) for the treatment of house dust mite (HDM)-induced allergic rhinitis, with or without conjunctivitis, to now include children ages 5 through 11 years, in addition to patients 12 through 65 years of age.ODACTRAconfirmed by positive in vitro testing for IgE antibodies to Dermatophagoides farinae or Dermatophagoides pteronyssinus house dust mites, or by positive skin testing to licensed house dust mite allergen extracts.
The Asia Pacific region is the fastest-growing region in the global skin allergy testing market, with a CAGR of 7.3% in 2024
The Asia-Pacific region is the fastest-growing market for skin allergy testing, driven by a surge in allergy prevalence, improving healthcare access, and growing awareness about dermatological health. Rapid urbanization, industrialization, and lifestyle changes in countries like China, India, and Japan have led to rising cases of atopic dermatitis, urticaria, contact dermatitis, and food-related skin allergies, particularly in children and young adults.
At the same time, increasing occupational exposure to chemicals, adhesives, and metals in industries across India and Southeast Asia has boosted demand for patch testing, especially as awareness grows about work-related skin conditions. Global players are expanding their footprint in APAC, with ALK-Abelló and Staller genes Greer supplying standardized allergen extracts and prick test kits, while Chemo technique Diagnostics provides region-specific patch test panels covering local allergens like tropical plants and food proteins.
Europe Skin Allergy Testing Market
In Europe, the skin allergy testing market is experiencing strong growth, fueled by rising allergy prevalence, stringent regulatory standards, and widespread adoption of standardized diagnostic tools. Allergic conditions such as contact dermatitis, atopic eczema, and food allergies are increasingly common across the region, partly due to high exposure to industrial chemicals, cosmetics, and environmental allergens like pollen. This has made patch testing particularly important in European dermatology, where products like the TRUE Test panels (CE-marked) and the Chemo technique Diagnostics allergen series are extensively used to detect delayed hypersensitivity reactions.
Similarly, skin prick testing remains a staple in allergy clinics for respiratory and food allergies, supported by CE-marked allergen extracts from companies like Staller genes Greer, headquartered in Europe and playing a pivotal role in supplying standardized reagents. Recent clinical guidelines from the European Academy of Allergy and Clinical Immunology (EAACI) further reinforce skin prick and patch tests as frontline diagnostic tools, ensuring their consistent integration in clinical practice. Together, the combination of regulatory drivers, clinical guidelines, and established manufacturers makes Europe one of the most dynamic regions for skin allergy testing growth.
Competitive Landscape
Top companies in the skin allergy testing market include Thermos Fisher Scientific Inc., Staller genes Greer, Siemens Healthiness, Allergy Therapeutics, ALK, Chemo technique MB Diagnostics AB, HAL Allergy B.V., Inmunotek, and HYCOR Biomedical, among others.
Market Scope
Metrics | Details | |
CAGR | 7.1% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Test Type | Prick Test, Patch Test, Intradermal Test, and others |
End-User | Hospitals, Specialist Clinics, Diagnostic Laboratories, and Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America and the Middle East & Africa | |
The global skin allergy Testing Market report delivers a detailed analysis with 51 key tables, more than 43visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more medical imaging-related reports, please click here