Allergy Treatment Market Size
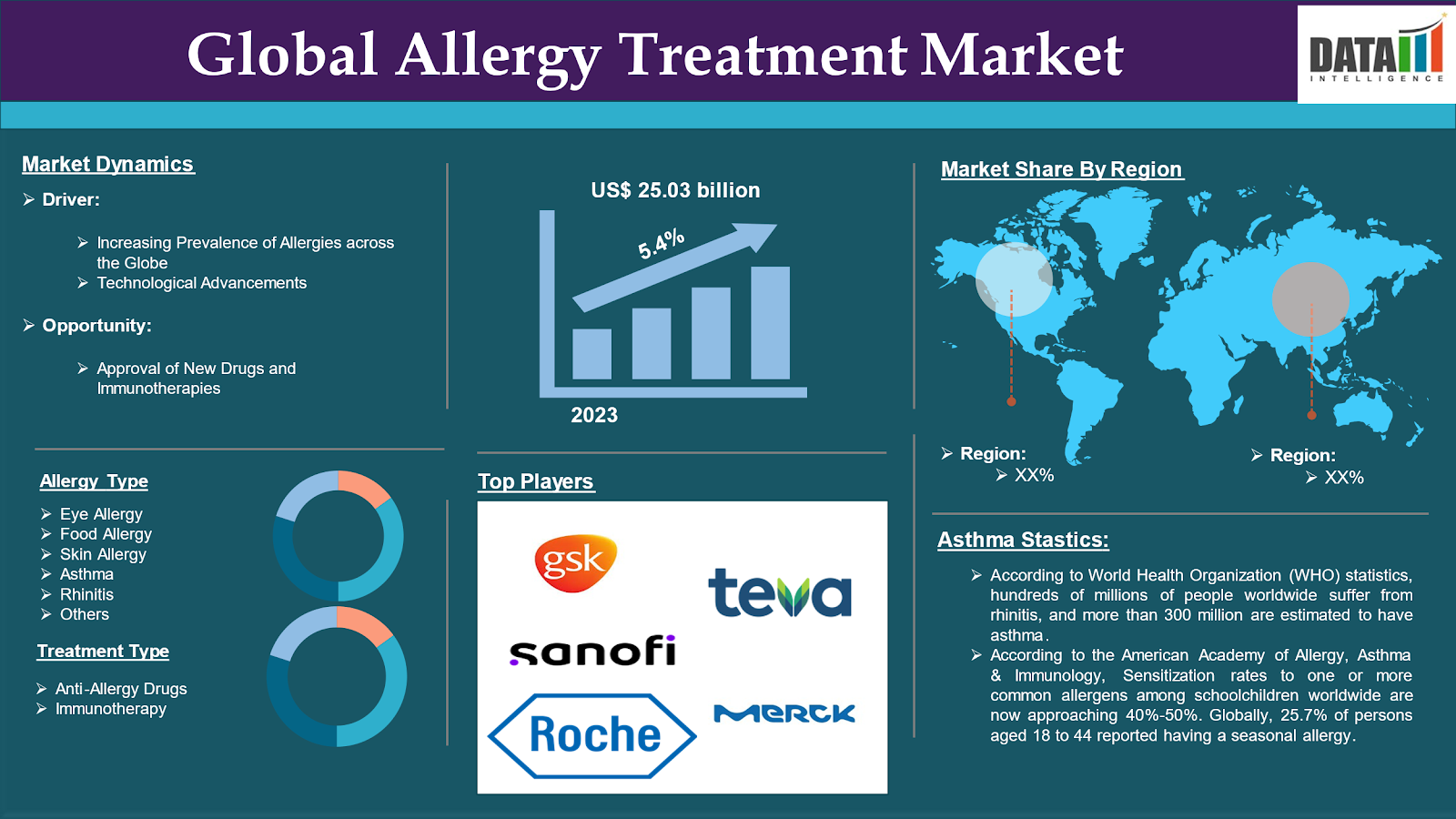
Global Allergy Treatment Market reached US$ 25.03 billion in 2023 and is expected to reach US$ 38.04 billion by 2031, growing at a CAGR of 5.4% during the forecast period 2024-2031.
Allergy is a hypersensitive reaction of the immune system to normally harmless chemicals known as allergens. These reactions arise when the immune system incorrectly perceives these harmless chemicals as threats, resulting in an excessive immunological response. Allergy treatment includes a number of treatments for managing symptoms and lowering the severity of allergic reactions. The primary approaches include allergen avoidance, usage of medication, and immunotherapy.
The increasing prevalence of allergies across the globe drives the market over the forecast period. Due to increasing prevalence, the demand for the treatment increases which propels the market. For instance, according to the Global Allergy and Airways Patient Platform, The global prevalence of allergy disorders is rapidly increasing in both developed and developing countries. The global prevalence of allergic disorders has steadily increased, with approximately 30-40% of the world's population now suffering from one or more allergic conditions.
For more details on this report - Request for Sample
Market Dynamics: Drivers
Increasing prevalence of allergies across the globe
The global allergy treatment market is majorly driven by the increasing prevalence of allergies. Allergic disorders are on the rise worldwide, including respiratory allergies (such as asthma and allergic rhinitis), food allergies, and skin allergies. This surge can be attributed to environmental causes such as increased pollution, urbanization, and lifestyle changes that expose people to more allergens.
Moreover, according to World Health Organization (WHO) statistics, hundreds of millions of people worldwide suffer from rhinitis, and more than 300 million are estimated to have asthma, reducing the quality of life for these people and their families and negatively impacting society's socioeconomic welfare. According to the American Academy of Allergy, Asthma & Immunology, Sensitization rates to one or more common allergens among schoolchildren worldwide are now approaching 40%-50%. Globally, 25.7% of persons aged 18 to 44 reported having a seasonal allergy.
Moreover, according to the World Allergy Organization Journal, allergic rhinitis, also known as hay fever, is a significant public health concern worldwide, affecting both developed and developing countries. Globally, allergic rhinitis affects more than 400 million people, with prevalence rates between 10% and 30% among adults and over 40% among children. Due to the increasing prevalence of allergies, the demand for allergy treatment medication has increased and it drives the allergy treatment market.
Side effects associated with allergy treatment drugs
Factors such as side effects associated with allergy treatment drugs are expected to hamper the market. The antihistamines cause side effects such as drowsiness, dry mouth, blurred vision, difficulty urinating, headaches, dizziness, feeling sick, and diarrhea. The decongestants cause side effects such as trouble sleeping, headaches, increased blood pressure, and irritability. The majority of drug allergies are treatable, although they can occasionally cause severe asthma, anaphylaxis, or death. These side effects hamper the market and act as a restraint for the allergy treatment market.
Market Segment Analysis
The global allergy treatment market is segmented based on allergy type, treatment type, distribution channel, and region.
Allergy Type:
Asthma is expected to dominate the global allergy treatment market share.
The asthma segment holds a major portion of the global allergy treatment market share and is expected to continue to hold a significant portion of the global allergy treatment market share during the forecast period. The increasing prevalence of asthma makes asthma the most dominating segment in the global allergy treatment market. As the asthma prevalence increases, the demand for treatment medication increases and leads to market growth.
For instance, according to the Global Initiative for Asthma, Asthma is one of the most common chronic non-communicable diseases, affecting over 260 million people and accounting for over 450000 deaths annually worldwide, the majority of which are preventable.
Rhinitis segment is fastest growing segment in global allergy treatment market.
The rhinitis segment is the fastest-growing segment in the global allergy treatment market share during the forecast period. The gradual increase of rhinitis these days makes rhinitis the fastest-growing segment in the global allergy treatment market.
For instance, according to the World Allergy Organization Journal, allergic rhinitis affects more than 400 million people, with prevalence rates between 10% and 30% among adults and over 40% among children.
Treatment Type:
Anti-allergy drugs segment is expected to dominate the global allergy treatment market share.
The integrated monitoring systems segment holds a major portion of the global allergy treatment market share and is expected to continue to hold a significant portion of the global allergy treatment market share during the forecast period.
Anti-allergy drugs are used to treat symptoms caused by allergic reactions. These reactions happen when the immune system overreacts to normally harmless chemicals called allergens, which include pollen, pet dander, and certain foods. When an allergen is encountered, the immune system releases molecules like histamine, causing symptoms such as sneezing, itching, and nasal congestion. The anti-allergy drugs are expected to dominate the market due to their versatility and efficiency in reducing allergies.
Immunotherapy segment is the fastest growing segment in the global allergy treatment market share.
The immunotherapy features segment is the fastest growing segment in the global allergy treatment market share. The increasing awareness among people regarding immunotherapy and increased research activities make immunotherapy the fastest growing segment.
Immunotherapy has emerged as a key strategy in allergy treatment, with the goal of altering the immune system's response to allergens substances that cause allergic reactions. Unlike standard allergy drugs, which only treat symptoms, immunotherapy aims to target the root problem by gradually desensitizing the immune system to specific allergens.
For instance, in October 2024, Curex announced the launch of its unique at-home Sublingual Immunotherapy (SLIT) program for food allergies. This new service seeks to enable people with food allergies to safely and conveniently complete clinical desensitization at home.
Market Geographical Share
North America is expected to hold a significant position in the global allergy treatment market share.
North America region is expected to hold the largest market share over the forecast period. The rising incidence of chronic diseases, and recent launches, in this region, help to propel the market. The presence of top players, the increasing prevalence of allergies, and recent launches and approvals in the North American market helped to propel the market in this region.
For instance, in August 2024, The United States Food and Drug Administration (FDA) approved the first nasal spray for treating anaphylaxis, a severe and life-threatening allergic reaction that requires prompt medical attention, as well as an injection of epinephrine, a hormone shot that fights the allergen. The increasing prevalence of allergies is also one of the major driving factors. For instance, according to the American Academy of Allergy, Asthma & Immunology, roughly 7.8% of people 18 and over in the U.S. have hay fever.
Moreover, according to the American College of Allergy, Asthma & Immunology, asthma and allergic illnesses, such as allergic rhinitis (hay fever), food allergies, and eczema, affect people of all ages throughout the United States. Asthma affects more than 24 million individuals in the United States, including over 4.6 million children.
Asia Pacific is growing at the fastest pace in the global allergy treatment market
Asia Pacific region is expected to grow at the fastest pace over the forecast period. The increasing prevalence of allergies and increased research and development activities of various products help drive the market in this region.
For instance, according to the National Institute of Health, Asthma is estimated to affect 37.5 million people in India, and recent research shows that the prevalence of allergic rhinitis and asthma is increasing. Approximately 40-50% of pediatric asthma cases in India are uncontrolled or severe. Due to increases in allergies, the demand for allergy treatment is increasing day by day making this region the fastest-growing region in the forecast period.
Market Segmentation
By Allergy Type
- Eye Allergy
- Food Allergy
- Skin Allergy
- Asthma
- Rhinitis
- Others
By Treatment Type
- Anti-Allergy Drugs
- Antihistamines
- Corticosteroids
- Leukotriene Inhibitors
- Others
- Immunotherapy
- Subcutaneous Immunotherapy
- Sublingual Immunotherapy
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- U.K.
- France
- Spain
- Italy
- Rest of Europe
- South America
- Brazil
- Argentina
- Rest of South America
- Asia-Pacific
- China
- India
- Japan
- South Korea
- Rest of Asia-Pacific
- Middle East and Africa
Market Competitive Landscape
The major global players in the global allergy treatment market include GlaxoSmithKline plc, Teva Pharmaceutical Industries Ltd., Stallergenes Greer, Allergy Therapeutics, Sanofi, Sumitomo Pharma Inc., Johnson & Johnson Services Inc., Merck & Co. Inc., F. Hoffmann-La Roche Ltd., AbbVie Inc. among others.
Emerging Players
Angany Inc., DBV Technologies, and Vedanta Biosciences, Inc. among others.
Metrics | Details | |
CAGR | 5.4% | |
Market Size Available for Years | 2022-2031 | |
Estimation Forecast Period | 2024-2031 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Allergy Type | Eye Allergy, Food Allergy, Skin Allergy, Asthma, Rhinitis, Others |
Treatment Type | Anti-Allergy Drugs, Immunotherapy | |
Distribution Channel | Hospital Pharmacy, Retail Pharmacy, Online Pharmacy | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and Middle East & Africa | |
Key Developments
- In February 2024, the U.S. Food and Drug Administration approved Xolair (omalizumab) injection for immunoglobulin E-mediated food allergy in certain adults and children 1 year or older for the reduction of allergic reactions (Type I), including reducing the risk of anaphylaxis, that may occur with accidental exposure to one or more foods.
Why Purchase the Report?
- To visualize the global allergy treatment market segmentation based on allergy type, treatment type, distribution channel, and region, as well as understand key commercial assets and players.
- Identify commercial opportunities by analyzing trends and co-development
- Excel data sheet with numerous data points of global allergy treatment market level with all segments.
- PDF report consists of a comprehensive analysis after exhaustive qualitative interviews and an in-depth study.
- Product mapping is available in Excel consisting of key products of all the major players.
The global allergy treatment market report would provide approximately 51 tables, 54 figures, and 181 Pages.
Target Audience 2024
- Manufacturers/ Buyers
- Industry Investors/Investment Bankers
- Research Professionals
- Emerging Companies