Rare Neuromuscular Disorders Market Size & Industry Outlook
The global rare neuromuscular disorders market size reached US$ 6.13 Billion in 2024 from US$ 5.72 Billion in 2023 and is expected to reach US$ 11.91 Billion by 2033, growing at a CAGR of 7.8% during the forecast period 2025-2033. The market is experiencing rapid growth, driven by advances in gene and RNA-based therapies, improved genetic testing, and rising diagnosis rates. Approved treatments such as Spinraza (nusinersen), Zolgensma (onasemnogene abeparvovec), and Evrysdi (risdiplam) for spinal muscular atrophy (SMA) along with Exondys 51, Vyondys 53, Amondys 45, and Duvyzat (givinostat) for Duchenne muscular dystrophy (DMD), illustrate the market’s transition toward targeted molecular approaches. Ongoing innovation, strong regulatory support for orphan drugs, and evolving outcomes-based reimbursement models are expected to further accelerate global market growth.
Key Market Highlights
- North America dominates the rare neuromuscular disorders market with the largest revenue share of 44.17% in 2024.
- The Asia Pacific is the fastest-growing region and is expected to grow at the fastest CAGR of 7.5% over the forecast period.
- Based on therapeutic type, the gene therapy segment led the market with the largest revenue share of 40.13% in 2024.
- The major market players in the rare neuromuscular disorders market are Biogen, Genentech USA, Inc., Novartis AG, Sarepta Therapeutics, Inc., PTC Therapeutics, Inc., Catalyst Pharmaceuticals, Inc., NMD PHARMA A/S, and ActioBio, among others

Market Dynamics
Drivers: Strong orphan drug and regulatory incentives are significantly driving the rare neuromuscular disorders market growth
Strong orphan drug and regulatory incentives are a key force accelerating growth in the rare neuromuscular disorders market by lowering development risks and improving commercial viability for small patient populations. Global frameworks like the U.S. and Europe Orphan Drug Designation are major catalysts for market growth, as they provide critical incentives such as seven years of market exclusivity, tax credits for clinical testing, and FDA fee reductions. These benefits make developing high-cost therapies for ultra-rare diseases financially viable, attracting both biotech startups and major pharmaceutical companies. As a result, more gene and RNA-based treatments are advancing rapidly through pipelines, significantly expanding therapeutic options and accelerating overall market growth.
For instance, in August 2025, Keros Therapeutics, Inc., a clinical-stage biopharmaceutical company focused on developing and commercializing novel therapeutics to treat a wide range of patients with disorders that are linked to dysfunctional signaling of the transforming growth factor-beta (TGF-ß) family of proteins, announced the U.S. Food and Drug Administration granted Orphan Drug designation for KER-065 for the treatment of Duchenne muscular dystrophy.
Similarly, in September 2025, Cure Rare Disease, a 501(c)(3) nonprofit biotechnology company developing genetic therapies for ultra-rare neuromuscular diseases, announced that the U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation (ODD) for its investigational therapy, CRD-003, for the treatment of congenital muscular dystrophy caused by biallelic mutations in the FKRP gene (Limb-Girdle Muscular Dystrophy Type R9, LGMD2i/R9).
Additionally, in January 2025, NMD Pharma A/S, a clinical-stage biotech company dedicated to developing novel and improved treatments for patients living with neuromuscular diseases, announced that the U.S. Food and Drug Administration (FDA) granted orphan drug designation (ODD) for NMD670, a novel, oral, small molecule inhibitor of the skeletal muscle-specific chloride ion channel ClC-1, for the treatment of Charcot-Marie-Tooth disease (CMT).
Restraints: Long-term safety and durability concerns are hampering the growth of the market
Long-term safety and durability concerns are a significant restraint on the growth of the rare neuromuscular disorders market, particularly for gene and RNA-based therapies. While these treatments offer potential one-time or disease-modifying benefits, there is limited long-term clinical data on how durable their effects truly are and whether re-dosing will be feasible. For instance, AAV-based gene therapies like Zolgensma (onasemnogene abeparvovec) have shown remarkable early outcomes in SMA, but questions remain about sustained efficacy beyond several years and risks of immune response or liver toxicity.
Similarly, antisense oligonucleotides (ASOs) used in DMD and SMA require repeated administration, and their long-term safety in pediatric patients is still under evaluation. Regulators often mandate post-marketing surveillance and 15-year follow-up studies, delaying broader adoption and increasing costs for developers. These uncertainties make payers cautious about reimbursing high-cost, potentially non-permanent therapies. Consequently, despite strong scientific promise, ongoing concerns over durability, immunogenicity, and re-treatment limitations continue to temper investor confidence and slow the overall expansion of the rare neuromuscular disorders therapeutics market.
For more details on this report – Request for Sample
Rare Neuromuscular Disorders Market, Segmentation Analysis
The global rare neuromuscular disorders market is segmented based on disease type, therapeutic type, distribution channel, and region.
Therapeutic Type: The gene therapy segment is dominating and the fastest-growing in the rare neuromuscular disorders market with a 40.13% share in 2024

Gene therapy has emerged as both the dominant and one of the fastest-growing segments within the rare neuromuscular disorders market because it delivers one-time, disease-modifying and potentially curative outcomes that translate into very high per-patient revenues and intense commercial focus, the clear posterchild is Zolgensma (onasemnogene abeparvovec) for SMA, the first AAV-based approval that reshaped payer willingness to finance curative pricing. Regulatory momentum and surrogate-endpoint pathways have accelerated launches in adjacent indications, too, for instance, Elevidys (delandistrogene moxeparvovec) for Duchenne was approved and its label expanded in recent years, demonstrating that gene replacement for muscle diseases can cross regulatory hurdles.
These approvals create immediate market value and validate platform investment, which drives outsized R&D spending and rapid pipeline progression in AAV and other delivery modalities. The combination of newborn screening expansion, payer experiments with annuity/outcomes contracts, and iterative improvements in vector design and delivery means gene therapy both currently drives the largest share of revenue and is expected to remain the fastest-growing, high-value frontier in the rare neuromuscular disorders market for the remainder of the decade.
Rare Neuromuscular Disorders Market, Geographical Analysis
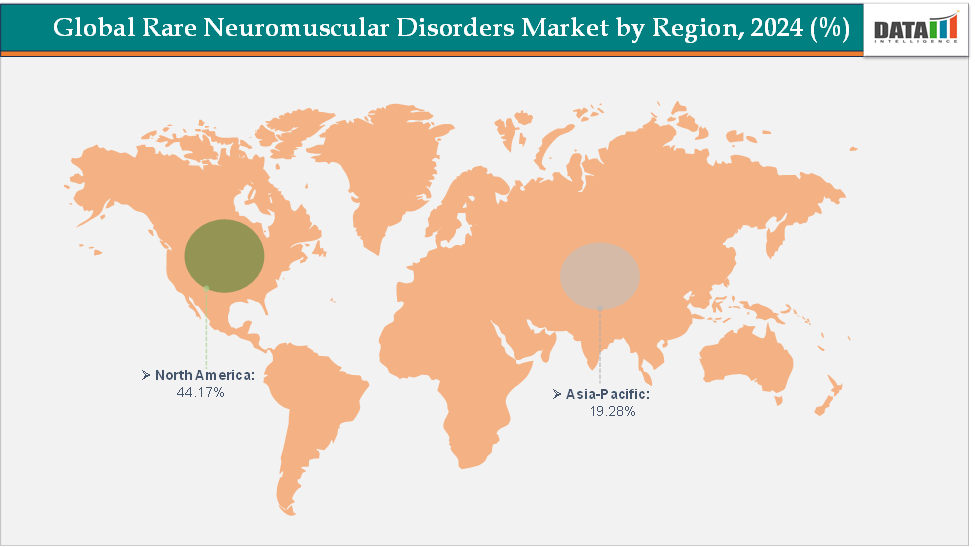
North America is expected to dominate the global rare neuromuscular disorders market with a 44.17% in 2024
North America is expected to dominate the global rare neuromuscular disorders market due to its strong presence of leading biopharmaceutical companies, advanced healthcare infrastructure, and favorable regulatory frameworks such as the U.S. Orphan Drug Act that incentivize innovation. The region also benefits from high diagnosis rates, early adoption of gene and RNA-based therapies, and extensive insurance coverage for high-cost orphan drugs, driving the largest market share globally.
US Rare Neuromuscular Disorders Market Trends
The US market for rare neuromuscular disorders is positioned to dominate globally due to a combination of early regulatory breakthroughs, high‐diagnosis rates and strong infrastructure supporting genetics‐based care. For instance, in the US, the estimated prevalence of Spinal Muscular Atrophy (SMA) is about 1 in 14,694 newborns after screening nearly 6.25 million infants across 30 states. The Zolgensma gene therapy (onasemnogene abeparvovec-xioi) was approved by the U.S. Food & Drug Administration (FDA) in May 2019 as the first gene therapy for SMA, targeting patients under age 2.
Similarly, in June 2023, the FDA approved Elevidys (delandistrogene moxeparvovec-rokl) as the first gene therapy for Duchenne Muscular Dystrophy (DMD) for certain ambulatory children 4-5 years old, and in 2024 expanded it to non-ambulatory patients 4+ years old. The US also leads in newborn screening programs, specialist treatment centers and the reimbursement environment for high-cost orphan therapies, all of which enable faster uptake. With a mature pipeline and high payer willingness, the US is both generating early high revenues and setting the global standard for launches, making it the leading region in the rare neuromuscular disorders market.
The Asia Pacific region is the fastest-growing region in the global rare neuromuscular disorders market, with a CAGR of 7.5% in 2024
The Asia-Pacific (APAC) region is the fastest-growing area in the global rare neuromuscular disorders market, driven by rapid advancements in genetic diagnostics, evolving orphan drug regulations, and increasing access to innovative therapies. Countries such as Japan, China, and South Korea are actively strengthening their rare disease frameworks, especially with Japan’s orphan drug designation and China’s approval of AGAMREE (vamorolone) for DMD in 2024, highlighting this progress.
Regulatory agencies like the NMPA and PMDA are now offering priority reviews and accelerated approvals, encouraging faster introduction of novel gene and RNA therapies. For instance, in September 2025, Dyne Therapeutics, Inc. announced that the Ministry of Health, Labour and Welfare (MHLW) in Japan granted Orphan Drug designation for DYNE-251 in individuals with Duchenne muscular dystrophy (DMD) who have mutations in the DMD gene that are amenable to exon 51 skipping. DYNE-251 is being evaluated in the ongoing Phase 1/2 DELIVER global clinical trial.
Europe Rare Neuromuscular Disorders Market Trends
Europe’s rare neuromuscular disorders market has grown into a leading and increasingly dynamic region thanks to a strong regulatory ecosystem, active national HTA pathways, and an expanding clinical and diagnostic infrastructure that together accelerate both approvals and real-world uptake. The European Commission and EMA have granted landmark authorizations for transformative SMA and DMD medicines Spinraza (nusinersen) and Evrysdi (risdiplam) have been available in the EU for several years, and Zolgensma received a conditional marketing authorisation in May 2020 that was later converted to full approval establishing precedent for high-value gene and RNA therapies.
At the same time, the EU has recently welcomed new treatment options for Duchenne AGAMREE (vamorolone) received EC approval in December 2023 and Duvyzat (givinostat) obtained conditional authorisation in June 2025 broadening therapeutic choices and reinforcing Europe’s role in bringing novel neuromuscular treatments to patients.
Europe’s policy tools such as orphan designation, PRIME/accelerated pathways, and conditional marketing authorisations, incentivize industry investment and faster filings, while well-established networks of neuromuscular centres and newborn screening pilots increase diagnosis and referral rates critical for early treatment uptake and robust post-marketing evidence generation. However, the region also exemplifies the sector’s complexity with high-profile regulatory caution around some gene therapies, showing that rigorous European scrutiny can both protect patients and temporarily slow commercial rollouts.
Competitive Landscape
Top companies in the rare neuromuscular disorders market include Biogen, Genentech USA, Inc., Novartis AG, Sarepta Therapeutics, Inc., PTC Therapeutics, Inc., Catalyst Pharmaceuticals, Inc., NMD PHARMA A/S, and ActioBio, among others.
Market Scope
| Metrics | Details | |
| CAGR | 7.8% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Disease Type | Spinal Muscular Atrophy (SMA), Duchenne Muscular Dystrophy (DMD), Myasthenia Gravis, Charcot-Marie-Tooth (CMT), and Others |
| Therapeutic Type | Gene Therapy, Antisense Oligonucleotides, Cell Therapy, Disease-Modifying Agents, RNA Therapeutics, and Others | |
| Distribution Channel | Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies | |
| Regions Covered | North America, Europe, Asia-Pacific, South America and the Middle East & Africa | |
The global rare neuromuscular disorders market report delivers a detailed analysis with 56 key tables, more than 55 visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more pharmaceuticals-related reports, please click here