Personalized Cancer Vaccines Market Size
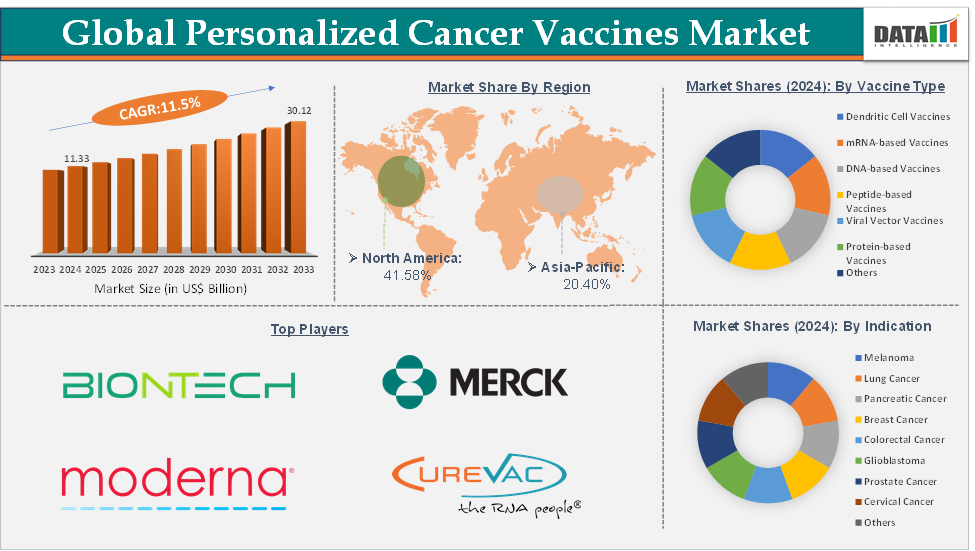
The global personalized cancer vaccines market size reached US$ 11.33 Billion in 2024 from US$ 10.21 Billion in 2023 and is expected to reach US$ 30.12 Billion by 2033, growing at a CAGR of 11.5% during the forecast period 2025-2033.
Overview
The personalized cancer vaccines market is revolutionizing global cancer treatment by shifting from one-size-fits-all therapies to customized immunotherapies that target each patient's unique tumor mutations. Powered by mRNA and neoantigen technologies, these vaccines stimulate the immune system to recognize and destroy cancer cells more precisely. This innovation is driving faster clinical responses, reduced side effects, and long-term remission potential, particularly in hard-to-treat cancers like melanoma and pancreatic cancer. As AI streamlines vaccine design, the approach is becoming increasingly scalable and globally accessible.
Executive Summary
Dynamics
Drivers:
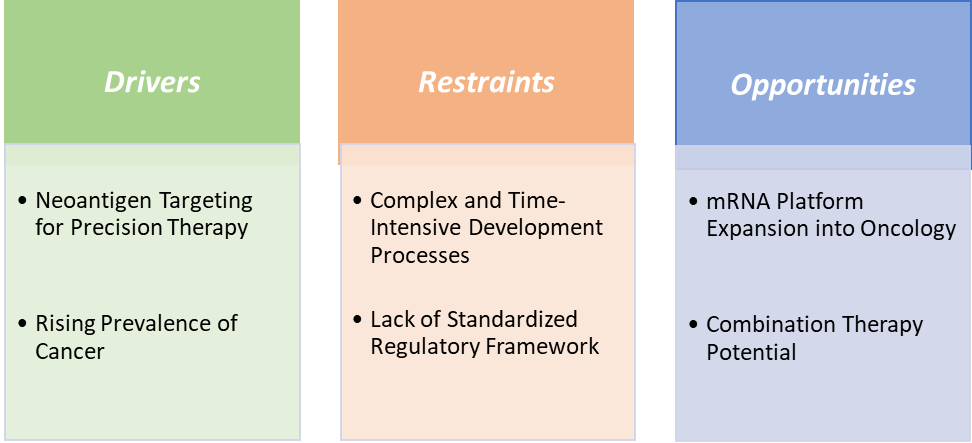
The rising prevalence of cancer is significantly driving the personalized cancer vaccines market growth
The rising global prevalence of cancer is significantly accelerating the growth of the personalized cancer vaccines market, as healthcare systems increasingly seek more effective, targeted treatment options. For instance, according to the National Institutes of Health, cancer is among the leading causes of death worldwide. In 2022, there were almost 20 million new cases and 9.7 million cancer-related deaths worldwide. By 2040, the number of new cancer cases per year is expected to rise to 29.9 million and the number of cancer-related deaths to 15.3 million. Additionally, according to the International Agency for Research on Cancer, in 2025, cancer incidence cases are projected to reach 21.3 million, and in 2030, the cases are estimated to reach 24.1 million.
This surge is intensifying the demand for therapies that go beyond the limitations of traditional chemotherapy and radiation, which often cause severe side effects and have variable success rates. Personalized cancer vaccines, designed using each patient’s unique tumor mutations (neoantigens), offer a highly targeted immune response that improves treatment precision and lowers toxicity.
Restraints:
A complex and time-intensive development process is hampering the growth of the personalized cancer vaccines market
Unlike traditional "one-size-fits-all" therapies, personalized cancer vaccines must be custom-designed for each patient, requiring tumor biopsy, genomic sequencing, neoantigen identification, and vaccine formulation, often taking 6 to 12 weeks or more per patient. This prolonged timeline is critical in aggressive cancers like glioblastoma or metastatic melanoma, where rapid treatment is vital.
Additionally, the need for sophisticated bioinformatics, GMP-certified facilities, and highly trained personnel drives up production costs and limits scalability. In clinical trials, these challenges often lead to patient dropouts or delays, affecting trial outcomes and regulatory timelines. Moreover, the regulatory pathway for personalized vaccines is still evolving, as agencies like the FDA must assess safety and efficacy on a patient-specific basis, adding further complexity. Smaller biotech firms, despite innovation, may lack the infrastructure to scale production quickly. As a result, while the science is promising, the operational demands of personalized vaccine development currently limit widespread adoption and slow down market expansion.
For more details on this report – Request for Sample
Segmentation Analysis
The global personalized cancer vaccines market is segmented based on vaccine type, indication, end-user, and region.
The mRNA-based vaccines segment from the vaccine type is dominating the personalized cancer vaccines market with a 33.05% share in 2024
mRNA-based vaccines have emerged as the dominant segment in the personalized cancer vaccines market due to their flexibility, speed of development, and strong clinical outcomes. Unlike traditional vaccines, mRNA-based approaches allow for the rapid encoding of patient-specific tumor neoantigens, making them ideal for personalized immunotherapy. The success of mRNA platforms during the COVID-19 pandemic validated their safety, scalability, and global manufacturing potential, accelerating their adoption in oncology.
For instance, Moderna and Merck’s mRNA-4157/V940 demonstrated a 44% reduction in the risk of recurrence or death in melanoma patients when combined with Keytruda. Similarly, BioNTech’s BNT122, developed with Genentech, is in advanced trials for solid tumors and pancreatic cancer. These vaccines leverage AI-driven antigen selection and high-speed sequencing, enabling faster turnaround times compared to dendritic cell or peptide-based vaccines.
Moreover, mRNA vaccines can be produced without using live cells or complex purification, simplifying the manufacturing process and reducing costs at scale. As a result, global pharma giants are heavily focusing on personalized mRNA vaccine pipelines, pushing this segment to the forefront. Their proven clinical efficacy, modularity, and commercial scalability make mRNA-based vaccines the leading force in the evolving personalized cancer vaccine landscape.
Geographical Share Analysis
North America is expected to dominate the global personalized cancer vaccines market with a 41.58% in 2024
North America is projected to lead the global personalized cancer vaccines market due to its advanced healthcare infrastructure, strong R&D ecosystem, and early adoption of cutting-edge immunotherapies. The region is driven by high cancer prevalence and favorable regulatory pathways like the FDA’s Breakthrough Therapy Designation granted to Moderna and Merck’s mRNA-4157/V940 vaccine for melanoma.
The U.S. dominates clinical trial activity, hosting the majority of the 80+ ongoing personalized vaccine trials across cancers such as lung, melanoma, and pancreatic. Major players like BioNTech, Moderna, Merck, and Gritstone Bio are headquartered or partnered in the region, accelerating innovation. Moreover, increasing investment in AI-based antigen selection and mRNA manufacturing capabilities positions North America as the frontrunner in commercializing and scaling personalized cancer vaccines globally.
Competitive Landscape
Top companies in the personalized cancer vaccines market include BioNTech SE, Merck & Co., Inc., Moderna, Inc., Gritstone Bio, Transgene, CureVac SE, Nouscom, and Evaxion A/S, among others.
Report Scope
Metrics | Details | |
CAGR | 11.5% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Vaccine Type | Dendritic Cell Vaccines, mRNA-based Vaccines, DNA-based Vaccines, Peptide-based Vaccines, Viral Vector Vaccines, Protein-based Vaccines and Others |
Indication | Melanoma, Lung Cancer, Pancreatic Cancer, Breast Cancer, Colorectal Cancer, Glioblastoma, Prostate Cancer, Cervical Cancer and Others | |
End-User | Hospitals, Cancer Research Centers, Biotechnology & Pharma Companies, Academic Institutions and Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America and the Middle East & Africa | |
The global personalized cancer vaccines market report delivers a detailed analysis with 56 key tables, more than 62 visually impactful figures, and 167 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more pharmaceutical-related reports, please click here