Eosinophilic Esophagitis (EoE) Treatment Market Overview
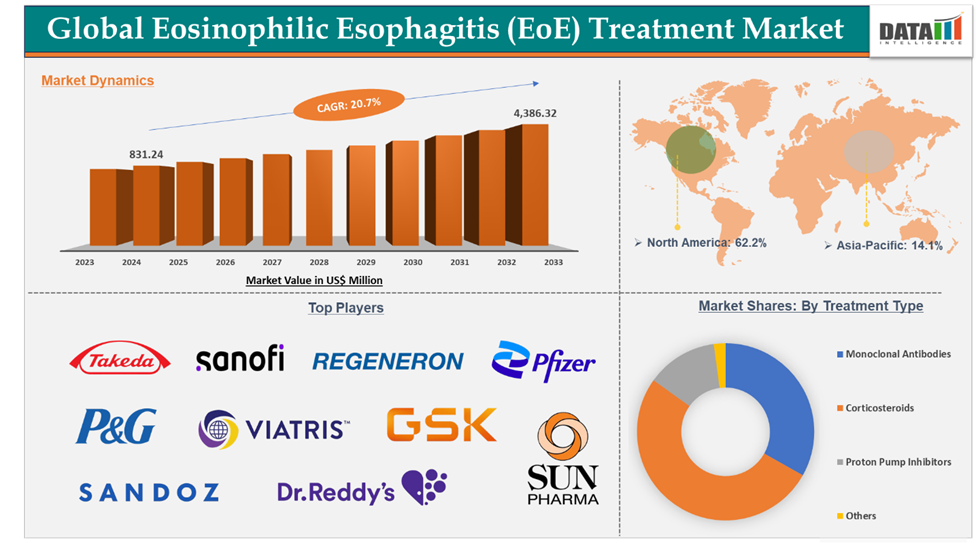
Eosinophilic Esophagitis (EoE) Treatment Market reached US$ 831.24 million in 2024 and is expected to reach US$ 4,386.32 million by 2033, growing at a CAGR of 20.7% during the forecast period 2025-2033.
The market for eosinophilic esophagitis (EoE) treatment is experiencing lucrative growth driven by the rising incidence and prevalence of the condition, rising product development activities, and regulatory approvals. Among various treatment types, corticosteroids like budesonide and fluticasone propionate are widely used. The monoclonal antibodies are the fast-growing treatment options for EoE, with Dupilumab (Dupixent) being the first mAb to be approved for the condition. There are several monoclonal antibodies in clinical development, which are expected to make market entry in the forecast period and drive the overall market growth.
Executive Summary
For more details on this report – Request for Sample
Eosinophilic esophagitis (EoE) treatment Market Dynamics: Drivers & Restraints
The rising R&D activities and product approvals are driving the market growth
The market for eosinophilic esophagitis (EoE) is experiencing significant growth driven by the rising R&D activities and product approvals. Before the approval of dupilumab by the U.S. FDA, the treatment of EoE was given using generic proton pump inhibitors and corticosteroids. In May 2022, the U.S. FDA approved dupilumab (Dupixent) for the treatment of patients aged 12 years and older, weighing at least 40 kg. In January 2024, the U.S. FDA provided extended approval of the drug for children aged 1 year and older with eosinophilic esophagitis (EoE). This approval marks the availability of the drug to all patient population. Moreover, in February 2024, the U.S. FDA approved EOHILIA (budesonide oral suspension) for the treatment of people 11 years and older with eosinophilic esophagitis (EoE).
These two approvals mark a significant advancement in the EoE treatment landscape. There are several advanced therapies like monoclonal antibodies, which are successfully advancing in the clinical trials, and are expected to make market entry in the forecast period, and drive the overall market growth.
Adverse effects associated with the current standard of care treatment regimen may restrain the market growth
Patients diagnosed with eosinophilic esophagitis (EoE) often require long-term treatment with corticosteroids or proton pump inhibitors. Although several new drugs have been approved, they are associated with significant side effects, which can hinder their adoption and disrupt patient adherence. For instance, EOHILIA (budesonide oral suspension) can cause immunosuppression in patients and increase their susceptibility to infections. This drug can affect the growth in children. Moreover, it can cause erosive esophagitis, where the acid-related damage happens to the lining of the esophagus.
Eosinophilic esophagitis (EoE) treatment Market Segment Analysis
The global eosinophilic esophagitis (EoE) treatment market is segmented based on treatment type, route of administration, distribution channel, and region.
Corticosteroids in the treatment type segment accounted for 51.1% of the market share in 2024 in the global eosinophilic esophagitis (EoE) treatment market
The corticosteroids segment is expected to hold the largest market share over the forecast period. Usually, there is no cure for eosinophilic esophagitis (EoE), but corticosteroids such as budesonide and fluticasone propionate are most commonly used and are considered first-line therapeutics for the management of this condition. Corticosteroids constitute the mainstay of EoE treatment and are frequently used as first-line agents once EoE is confirmed. Corticosteroids decrease eosinophil mucosal migration by inhibiting cytokines, leading to reduced remodeling and tissue fibrosis. In recent times, a new version of corticosteroids was approved by the regulatory authorities, which was developed specifically to treat patients with eosinophilic esophagitis.
For instance, in February 2024, the U.S. Food and Drug Administration (FDA) approved budesonide oral suspension (EOHILIA) for the treatment of people 11 years and older with eosinophilic esophagitis (EoE). EOHILIA, developed by Takeda, is indicated for 12 weeks and is available in 2 mg/10 mL convenient, single-dose stick packs.
Corticosteroids continue to dominate the EoE treatment market, due to their higher adoption rate, lower cost, higher accessibility, and therapeutic efficacy. With the approval of the new formulation of budesonide, the segment is poised to experience significant growth in the forecast period.
Eosinophilic Esophagitis (EoE) Treatment Market Geographical Analysis
North America dominated the eosinophilic esophagitis (EoE) treatment market with the highest share of 62.2% in 2024
North America’s dominance in the global eosinophilic esophagitis market is attributable to factors like the higher prevalence of the condition. For instance, a recent study conducted by Hannah L Thel et al., published in the Clin Gastroenterol Hepatol. Journal in October 2024, has stated that the standardized prevalence of EoE among the U.S. population was 42.5/100,000, extrapolating to 472,380 cases.
Moreover, the presence of approved drugs, high accessibility of approved drugs to patients in the country through various distribution channels, and a higher proportion of revenue generated by the market players from the United States, due to higher spending and disposable income, positions the region as dominant.
Eosinophilic Esophagitis (EoE) Treatment Market Major Players
The major players in the Eosinophilic esophagitis (EoE) treatment market are Takeda Pharmaceutical Company Limited., Sanofi, Regeneron Pharmaceuticals, Inc., Procter & Gamble, Pfizer Inc., Viatris Inc., GSK Plc, Sun Pharmaceutical Industries Ltd., Sandoz AG, and Dr. Reddy’s Laboratories Ltd., among others.
Key Development
In May 2025, AQILION AB announced that the U.S. Food and Drug Administration (FDA) had approved the company’s Investigational New Drug Application (IND) for its drug candidate AQ280, which is being developed for patients with eosinophilic esophagitis.
In May 2025, Eupraxia Pharmaceuticals Inc. announced that its investigational product EP-104GI has shown positive outcomes in treated patients. EP-104GI is currently being evaluated in a Phase 1b/2a trial. Eupraxia has revealed that the patients who received the drug have shown sustained or improved treatment outcomes after nine months of therapy.
Market Scope
Metrics | Details | |
CAGR | 20.7% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Treatment Type | Corticosteroids, Monoclonal Antibodies, Proton Pump Inhibitors, and Others |
Route of Administration | Parenteral, Oral, and Topical | |
Distribution Channel | Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |