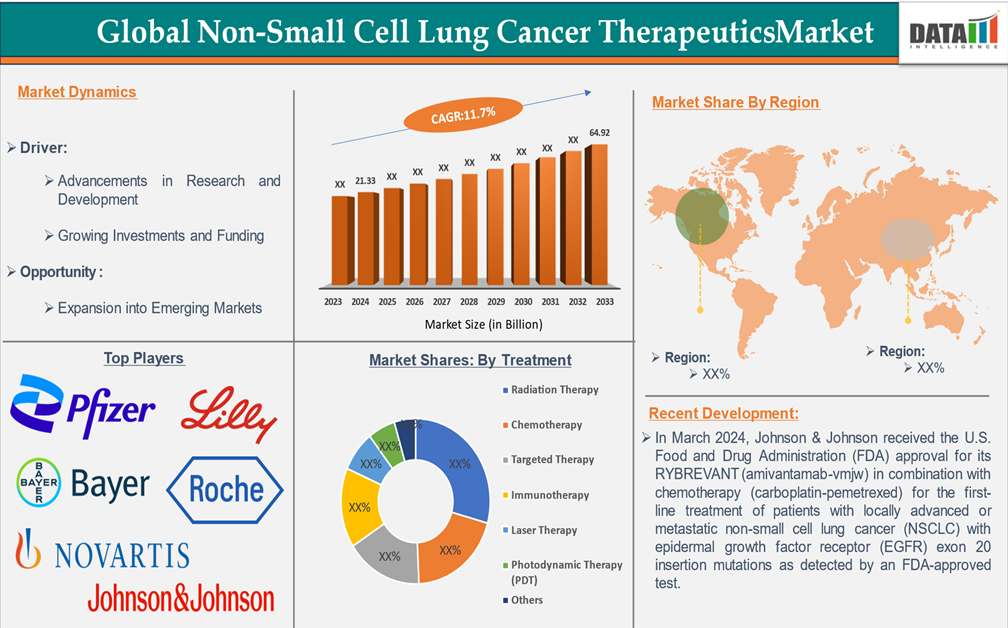
Market Size
The Global Non-Small Cell Lung Cancer Therapeutics Market reached US$21.33 billion in 2024 and is expected to reach US$64.92 billion by 2033, growing at a CAGR of 11.7% during the forecast period 2025-2033, according to DataM intelligence report.
Non-Small Cell Lung Cancer (NSCLC) represents the majority of lung cancer cases and encompasses several subtypes, including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Among these, adenocarcinoma is currently the most prevalent, accounting for nearly half of all lung cancer diagnoses. Previously, squamous cell carcinoma was more common, but recent trends show a shift, with SCC now increasingly detected in the peripheral regions of the lungs rather than solely originating from the central tracheobronchial tree, as per our analysis.
Executive Summary
For more details on this report – Request for Sample
Market Dynamics: Drivers & Restraints
Advancements in Research and Development
Advancements in research and development are expected to significantly drive the non-small cell lung cancer (NSCLC) therapeutics market by fostering innovation and enhancing treatment efficacy. Continuous investment in R&D has led to the development of novel therapies, particularly in targeted therapy and immunotherapy, which have shown promising results in improving patient outcomes.
Additionally, the increasing studies of cancer biology have facilitated the design of combination therapies that leverage multiple mechanisms of action, further improving therapeutic efficacy. For instance, in December 2022, GSK plc announced results from the PERLA phase II clinical trial investigating dostarlimab in combination with chemotherapy versus pembrolizumab in combination with chemotherapy as a first-line treatment for patients with metastatic non-squamous non-small cell lung cancer (NSCLC).
The growing pipeline of new drugs and the emergence of personalized medicine approaches are also contributing to market expansion, as they cater to the unique genetic profiles of patients. The development of innovative solutions with advanced research is contributing to the market growth. For instance, in December 2024, Datopotamab deruxtecan (Dato-DXd) was granted Breakthrough Therapy Designation (BTD) in the US for the treatment of adult patients with locally advanced or metastatic epidermal growth factor receptor-mutated (EGFRm) non-small cell lung cancer (NSCLC) with disease progression on or after treatment with an EGFR-tyrosine kinase inhibitor (TKI) and platinum-based chemotherapy.
Furthermore, increased funding from both the government and private sectors for cancer research is promoting innovation, leading to the introduction of advanced treatment options that are expected to meet the rising demand for effective NSCLC therapies. As a result, these advancements are likely to enhance treatment accessibility and improve overall survival rates, thereby propelling growth in the NSCLC therapeutics market.
High Costs of Treatment
The high cost of treatment is a significant barrier in the non-small cell lung cancer (NSCLC) market, limiting access to care for many patients and slowing market growth. Advanced therapies like immunotherapy, targeted drugs, and combination treatments offer promising outcomes, but they often come with hefty price tags that put financial strain on both patients and healthcare systems. In regions with limited healthcare funding or inadequate insurance coverage, these costs can lead to delayed diagnoses, skipped treatments, or a complete lack of access to potentially life-saving options.
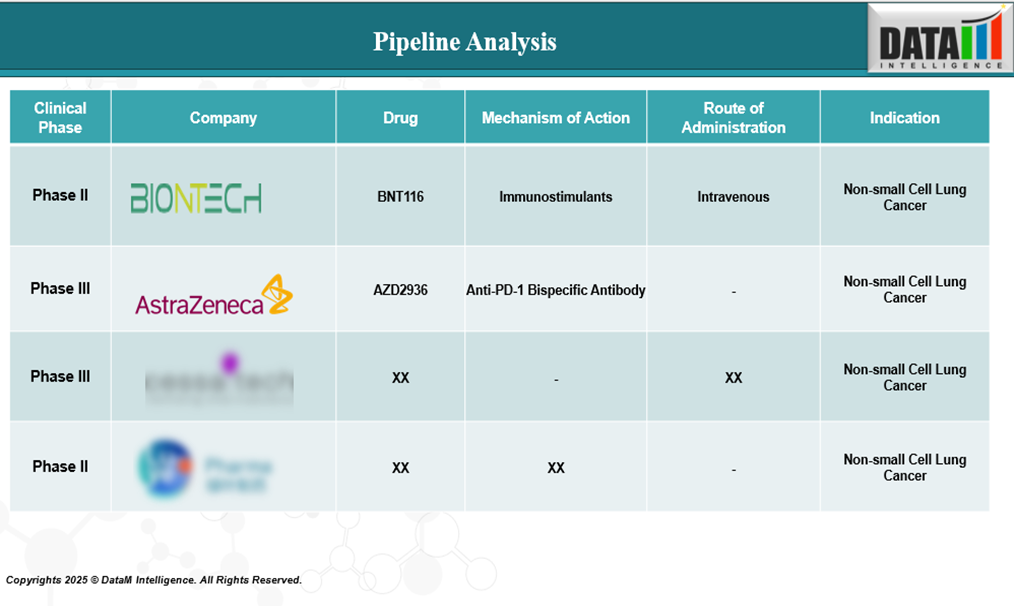
Pipeline Analysis:
Market Segment Analysis
The global non-small cell lung cancer therapeutics market is segmented based on type, treatment, and region.
Treatment:
Targeted therapy in the treatment segment is expected to dominate the non-small cell lung cancer therapeutics market.
The targeted therapy segment is poised to dominate the non-small cell lung cancer (NSCLC) therapeutics market due to its precision, efficacy, and growing adoption. Additionally, advancements in research and the development of new targeted agents continue to enhance their effectiveness, making them a preferred choice for treating advanced NSCLC. Companies are developing advanced targeted therapies.
For instance, in February 2024, Bayer announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy designation for BAY 2927088, a targetted drug intended for the treatment of adult patients with unresectable or metastatic non-small cell lung cancer (NSCLC) harboring activating HER2 (ERBB2) mutations who have previously received systemic therapy.
Market Geographical Analysis
North America is expected to dominate the non-small cell lung cancer therapeutics market.
North America is expected to dominate the non-small cell lung cancer (NSCLC) therapeutics market due to several key factors. The dominance is driven by a robust healthcare infrastructure, advanced medical technologies, and the presence of major pharmaceutical companies actively developing innovative therapies. The companies are receiving approvals for their innovative solutions, which are contributing to the market growth. For instance, in August 2024, Johnson & Johnson announced that the U.S. Food and Drug Administration (FDA) granted approval for its combination treatment of RYBREVANT (amivantamab-vmjw) and LAZCLUZE (lazertinib) as a first-line therapy for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) exhibiting epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations, as identified by an FDA-approved test.
Companies in the region are increasingly investing in developing innovative therapeutics to address the disease. For instance, in March 2024, Merck announced that the Phase 3 KEYLYNK-006 trial assessing KEYTRUDA, Merck's anti-PD-1 therapy, in combination with maintenance LYNPARZA, a PARP inhibitor, did not achieve its dual primary endpoints of overall survival (OS) and progression-free survival (PFS) for the first-line treatment of certain patients with metastatic non-squamous non-small cell lung cancer (NSCLC).
Increased awareness regarding early diagnosis and treatment options has further contributed to market growth, as more patients seek timely interventions. The U.S. specifically plays a pivotal role in this landscape due to high smoking rates and air pollution contributing to lung cancer prevalence. With ongoing investments in research and development, North America is poised to maintain its leadership position in the NSCLC therapeutics market for the foreseeable future.
Global Market Players
The global market players in the non-small cell lung cancer therapeutics market include F. Hoffmann-La Roche Ltd, Mylan N.V., Teva Pharmaceutical Industries Ltd., Pfizer Inc., Novartis AG, Bayer AG, Eli Lilly and Company, Merck & Co., Inc., AstraZeneca, Johnson & Johnson Services, Inc., among others.
Scope
| Metrics | Details | |
| CAGR | 11.7% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Type | Adenocarcinoma, Squamous Cell Carcinoma, Large Cell Carcinoma, Adenosquamous Carcinoma, Sarcomatoid Carcinoma | |
| Segments Covered | Treatment | Radiation therapy, Chemotherapy, Targeted therapy, Immunotherapy, Laser therapy, Photodynamic Therapy (PDT), Others |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials, product pipelines, and forecasts upcoming pharmaceutical advancements.
- Type Performance & Market Positioning: Analyzes product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: Covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyzes competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient Type delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global non-small cell lung cancer therapeutics market report will provide approximately 45 tables, 46 figures, and 180 pages.
Target Audience 2024
- Manufacturers: Pharmaceutical, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.