mRNA Vaccines and Therapeutics Market Size
mRNA Vaccines and Therapeutics market size reached US$ 46.32 Billion in 2024 and is expected to reach US$ 200.11 Billion by 2033, growing at a CAGR of 18.4% during the forecast period 2025-2033.
Global mRNA Vaccines and Therapeutics Market showed resilience and upward growth in its early stages, moving from US$ 42.62 billion in 2022 to US$ 44.33 billion in 2023.
The mRNA vaccines and therapeutics market is experiencing rapid growth, driven by the proven success of mRNA-based COVID-19 vaccines and the expanding potential of this technology across a range of therapeutic areas. Unlike traditional vaccines, mRNA vaccines work by instructing cells to produce proteins that mimic disease-causing agents, triggering targeted immune responses with greater precision and fewer side effects.
Key market drivers include accelerated R&D investment, technological advancements in delivery systems, and strong regulatory momentum following the pandemic. Opportunities lie in expanding into new indications such as influenza, RSV, HIV, and personalized oncology treatments.
Executive Summary
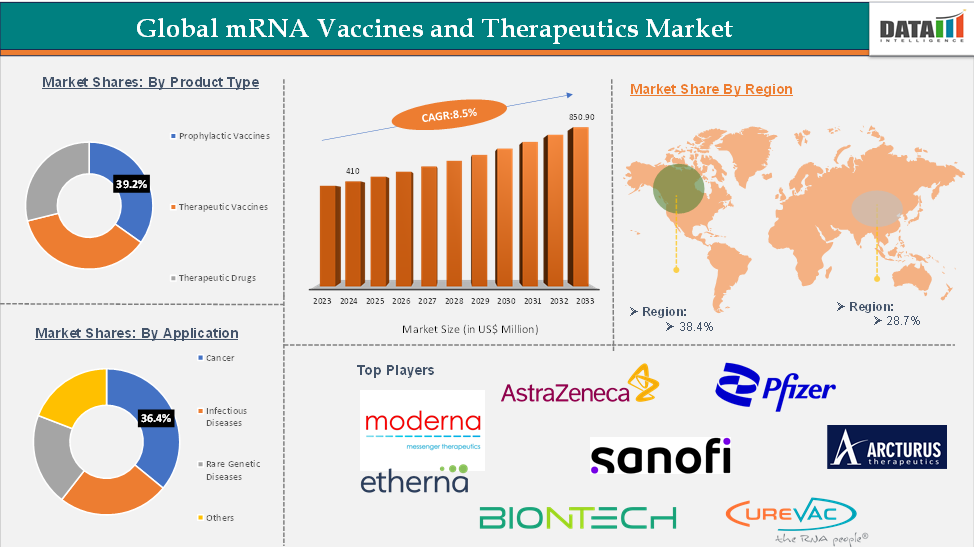
For more details on this report – Request for Sample
mRNA Vaccines and Therapeutics Market Dynamics: Drivers & Restraints
Growing Cases of Chronic Diseases are Expected to Drive the mRNA Vaccines and Therapeutics Market
The rising prevalence of chronic diseases such as cancer, cardiovascular diseases, and other autoimmune diseases is expected to drive the mRNA vaccines and therapeutics market growth. There is a growing demand for vaccines and therapeutics with the growing awareness of the increasing incidence of cancer cases. For instance, according to the National Cancer Institute in 2022, there were almost 20 million new cases and 9.7 million cancer-related deaths worldwide. By 2040, the number of new cancer cases per year is expected to rise to 29.9 million and the number of cancer-related deaths to 15.3 million.
As chronic diseases continue to place a significant strain on global healthcare systems, the demand for more effective treatments and preventative solutions is steadily increasing. mRNA technology, which has already proven its effectiveness in COVID-19 vaccines, holds great promise for developing targeted therapies for conditions like cancer and other chronic illnesses.
By utilizing mRNA’s ability to direct cells to produce specific proteins, researchers have the potential to create vaccines and therapeutics that address the root causes of diseases, offering more targeted treatments with fewer side effects. This growing need for advanced, personalized approaches to managing chronic diseases is driving the expansion of the mRNA-based therapeutics market, positioning it as one of the fastest-growing areas in modern medicine.
Competition from Alternative Vaccines and Therapeutics is Expected to Hinder the mRNA Vaccines and Therapeutics Market
Competition from alternative technologies could hinder the growth of the mRNA vaccines and therapeutics market by offering potentially safer or more established solutions. For instance, protein subunit vaccines, viral vector-based vaccines, and monoclonal antibodies are alternative platforms that have been used successfully in various treatments and may be preferred by some healthcare providers or patients due to their familiarity.
These alternatives may also present fewer concerns related to safety, storage requirements, or production challenges, making them more appealing in certain situations. As a result, the market share for mRNA-based products could face limitations, especially if these competing technologies prove more efficient, cost-effective, or widely accepted in the long run.
mRNA Vaccines and Therapeutics Market Segment Analysis
The global mRNA vaccines and therapeutics market is segmented based on product type, application, end-user, and region.
Component Type:
The prophylactic vaccines are expected to hold 77.3% of the global mRNA vaccines and therapeutics market
In 2022, the Prophylactic Vaccines segment represented one of the fastest-growing segments, reaching US$ 32.97 billion, and further increased to US$ 34.28 billion in 2023.
The prophylactic vaccines segment is poised to dominate the mRNA vaccines and therapeutics market, fueled by the proven success of mRNA technology in addressing infectious diseases, particularly with COVID-19 vaccines. The inherent speed and flexibility of mRNA platforms enable rapid development and scalable production, making them invaluable in responding to emerging health threats and pandemics. This capability has fostered strong confidence in mRNA vaccines, known for delivering robust, long-lasting immunity and their adaptability to new pathogens.
Beyond COVID-19, the development of mRNA vaccines for infectious diseases like influenza and HIV further positions this technology as a key solution for global health challenges. For instance, in June 2024, Moderna’s RSV vaccine, mRESVIA, received approval from the U.S. Food and Drug Administration (FDA) for use in adults aged 60 and over, marking the first FDA-approved mRNA vaccine for a disease other than COVID-19. This milestone represents a major advancement in vaccine technology and is expected to broaden the application of mRNA platforms across a wider range of diseases in the future.
As the approval and widespread adoption of mRNA-based vaccines continue to expand, the prophylactic vaccines segment is set to maintain a dominant position in the market, driven by the technology's effectiveness and its potential to address a wide range of infectious diseases.
mRNA Vaccines and Therapeutics Market Geographical Analysis
North America is expected to hold 42.8% of the global mRNA vaccines and therapeutics market
North America led the Global mRNA Vaccines and Therapeutics Market in 2022 with a market size of US$ 18.22 billion and expanded further to US$ 18.96 billion in 2023.
North America is expected to hold a significant position in the global mRNA vaccines and therapeutics market due to several key factors. Several pharmaceutical companies and biotech firms have been at the forefront of mRNA vaccine development, especially in response to the COVID-19 pandemic. These companies' expertise, along with substantial investment in mRNA technology, has positioned North America as a global leader in both research and commercialization of mRNA-based therapies.
For instance, in May 2024, Moderna Inc. received FDA approval for the mRNA RSV vaccine, which will be marketed as mRESVIA. The novel mRNA RSV vaccine is indicated to protect adults aged 60 years and older from lower respiratory tract disease (LRTD) caused by RSV infection. The approval was granted under a breakthrough therapy designation, marking Moderna’s second FDA-approved mRNA vaccine.
Additionally, North America benefits from a robust regulatory framework, with agencies such as the U.S. FDA and Health Canada offering significant support in the approval and distribution of mRNA vaccines and therapeutics. This conducive regulatory environment accelerates the approval process, ensuring faster market access and allowing companies to introduce innovative treatments with greater efficiency.
Moreover, the region has robust funding and clinical organizations involved in the development of several vaccines and therapeutics. Several clinical trials are being conducted in the region by companies and organizations, which increases the product portfolio and meets the rising demand for vaccines. For instance, in January 2025, ME Therapeutics Holdings Inc. announced that its first mRNA therapeutic candidate, developed in collaboration with NanoVation Therapeutics, has shown promising anti-cancer activity in a colorectal cancer mouse model. The company plans to follow up studies to validate the results and explore the candidate’s mechanism of action.
Additionally, in February 2023, Pfizer Inc. and BioNTech SE initiated a Phase 1/2 trial exploring the safety, tolerability, and immunogenicity of the companies’ mRNA vaccine candidates against shingles (also known as herpes zoster, or HZ), a debilitating disease caused by the varicella-zoster virus (VZV). Shingles affects millions of people around the world each year.
In December 2022, Moderna, Inc. and Merck announced that the Phase 2b KEYNOTE-942/mRNA-4157-P201 trial of mRNA-4157/V940, an investigational personalized mRNA cancer vaccine, in combination with KEYTRUDA, Merck’s anti-PD-1 therapy, demonstrated a statistically significant and clinically meaningful improvement in the primary endpoint of recurrence-free survival (RFS) versus KEYTRUDA alone for the adjuvant treatment of patients with stage III/IV melanoma following complete resection.
Asia-Pacific is expected to hold 18% of the global mRNA Vaccines and Therapeutics market
Asia-Pacific recorded strong growth, increasing from US$ 7.53 billion in 2022 to US$ 7.91 billion in 2023, supported by rising investments and growing demand in emerging economies like China and India.
The Asia-Pacific region is emerging as the fastest-growing market for mRNA vaccines and therapeutics, driven by robust R&D investment, supportive regulatory frameworks, and increasing public health initiatives.
Japan, in particular, is playing a pivotal role in this growth. For instance, in September 2024, Japan approved ARCT-154 (Kostaive), a self-amplifying mRNA COVID-19 vaccine developed by CSL and Arcturus Therapeutics, designed to protect against the JN.1 Omicron subvariant in adults aged 18 and older. This approval reflects the region’s growing commitment to next-generation vaccine technologies and underscores Asia-Pacific’s strategic position in advancing mRNA-based solutions for infectious diseases and beyond.
mRNA Vaccines and Therapeutics Market Top Companies
The top companies in the mRNA Vaccines and Therapeutics market include Azra Al, CureMetrix, Inc., ConcertAI, Immunai, MVision AI Inc., Paige AI, Inc., Mindpeak GmbH, Imagene AI Ltd., Predictive Oncology, and Tempus, among others.
Key Developments
- In January 2025, Esphera SynBio, a pre-clinical stage synthetic biology company, initiated a new project focused on improving the effectiveness of mRNA vaccines. Utilizing its proprietary technology, the company aims to engineer mRNA vaccines that direct patient cells to produce modified nanomedicines upon delivery, enhancing therapeutic impact and precision.
mRNA Vaccines and Therapeutics Market Scope
| Metrics | Details | |
| CAGR | 18.4% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Product Type | Prophylactic Vaccines, Therapeutic Vaccines, Therapeutic Drugs |
| Application | Cancer, Infectious Diseases, Rare Genetic Diseases, Others | |
| End-user | Hospitals & Clinics, Research Organization, Academic Institutes, Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global mRNA Vaccines and Therapeutics market report delivers a detailed analysis with 57 key tables, more than 46 visually impactful figures, and 168 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2024
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.