Influenza Vaccine Market Size
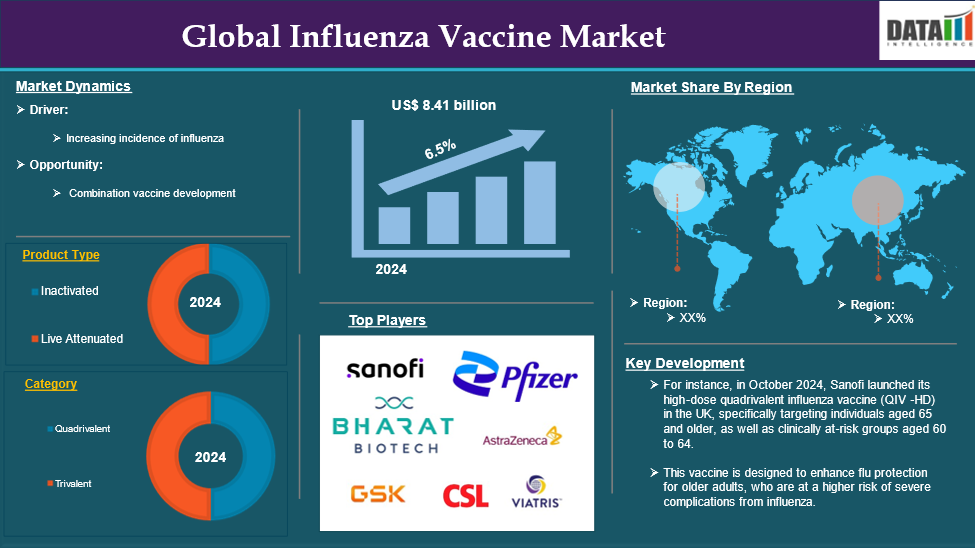
The Global Influenza Vaccine Market reached US$ 8.41 billion in 2024 and is expected to reach US$ 13.75 billion by 2032, growing at a CAGR of 6.5 % during the forecast period 2025-2032.
The global influenza vaccine market refers to the commercial sector dedicated to the development, production, and distribution of vaccines designed to prevent influenza, a contagious respiratory illness caused by influenza viruses. This market encompasses various types of vaccines, which are categorized primarily based on their formulation and the strains they target.
Types of vaccines include inactivated vaccines and live attenuated vaccines. Inactivated vaccines these vaccines are made from killed virus particles. They are the most commonly used type of influenza vaccine and are administered via injection. Inactivated vaccines stimulate an immune response without the risk of causing the disease, making them a safe choice for most populations.
Live attenuated vaccines these vaccines contain weakened forms of the influenza virus and are typically administered as a nasal spray. Live attenuated vaccines can provide a robust immune response because they closely mimic natural infection, but they may not be suitable for certain high-risk groups.
Influenza vaccines are also classified by their valency, which refers to the number of virus strains they protect against trivalent vaccines and quadrivalent vaccines. Trivalent vaccines protect against three strains of the influenza virus: two A strains (typically H1N1 and H3N2) and one B strain. Trivalent vaccines have been the traditional standard for flu vaccination.
Quadrivalent vaccines offer broader protection by targeting four strains: two A strains and two B strains (one from each lineage). This expanded coverage is particularly beneficial as it helps protect against more circulating strains during flu season, making quadrivalent vaccines increasingly preferred by healthcare providers and patients alike. These factors have driven the global influenza vaccine market expansion.
Executive Summary
For more details on this report – Request for Sample
Influenza Vaccine Market Dynamics: Drivers & Restraints
Increasing Incidence of Influenza
The increasing incidence of influenza is significantly driving the growth of the global influenza vaccine market and is expected to drive throughout the market forecast period.
The rising global prevalence of influenza significantly drives the demand for vaccines, as highlighted by estimates from the World Health Organization (WHO). According to WHO, seasonal influenza results in approximately 3 to 5 million cases of severe illness and 290,000 to 650,000 respiratory deaths each year. This substantial burden of disease underscores the urgent need for effective vaccination programs.
Similarly, according to the U.S. Centers for Disease Control and Prevention (CDC), influenza has resulted in an estimated 9.3 million to 41 million illnesses, 100,000 to 710,000 hospitalizations, and 4,900 to 51,000 deaths annually from 2010 to 2023. To combat this significant health burden, numerous FDA-approved vaccines are available each flu season to help prevent influenza.
The significant global burden of influenza in terms of severe illness and mortality drives the urgent need for effective vaccination programs. By increasing awareness of the importance of flu vaccines and implementing robust public health strategies, health authorities aim to reduce the incidence of influenza and its associated complications, ultimately improving population health outcomes worldwide.
Furthermore, major players in the industry have key initiatives that would propel this influenza vaccine market growth. For instance, in July 2024, a new initiative was launched to accelerate the development and accessibility of human avian influenza (H5N1) messenger RNA (mRNA) vaccine candidates, specifically targeting manufacturers in low- and middle-income countries (LMICs).
This project is led by Sinergium Biotech, an Argentinian manufacturer, and leverages the World Health Organization (WHO) and the Medicines Patent Pool (MPP) mRNA Technology Transfer Programme. All these factors demand the global influenza vaccine market.
Moreover, the rising demand for the growth of combination vaccine development contributes to the global influenza vaccine market expansion.
High Cost Associated with the Manufacturing Processes
The high cost associated with the manufacturing processes is a significant restraint in the global influenza vaccine market. This constraint arises from a variety of factors that contribute to the overall expenses involved in vaccine production, ultimately impacting both availability and pricing.
Establishing vaccine manufacturing facilities is capital-intensive, with costs ranging from $50 million to $500 million per antigen. The exact amount depends on the complexity of the design, automation, and necessary contamination control measures. Facilities designed to comply with current Good Manufacturing Practices (cGMP) require specialized equipment and infrastructure to ensure product safety and efficacy, driving up initial investment costs.
These costs stem from complex facility requirements, labor expenses, raw material prices, regulatory compliance needs, and lengthy investment recovery periods. Addressing these challenges through innovations in production technology and improved efficiency could help mitigate some of these financial burdens and enhance vaccine accessibility worldwide. Thus, the above factors could be limiting the global influenza vaccine market's potential growth.
Influenza Vaccine Market Segment Analysis
The global influenza vaccine market is segmented based on product type, category, route of administration, age group, end-user, and region.
Product Type:
The inactivated segment is expected to dominate the global influenza vaccine market share
The inactivated segment holds a major portion of the global influenza vaccine market share and is expected to continue to hold a significant portion of the global influenza vaccine market share during the forecast period.
The inactivated product segment of the global influenza vaccine market is vital for influenza prevention strategies, primarily consisting of inactivated influenza vaccines (IIVs). These vaccines are well-regarded for their safety and effectiveness. Inactivated influenza vaccines are formulated from viruses that have been killed or inactivated, ensuring they cannot cause disease. Typically, these vaccines contain antigens from multiple strains of the influenza virus, protecting against various circulating strains.
Inactivated influenza vaccines (IIVs) are recognized for their robust safety profile and effectiveness in preventing influenza-related illnesses. They stimulate an immune response without the risk of causing the disease, making them suitable for diverse populations, including children and the elderly. The production process for IIVs can be effectively scaled to meet global demand, especially during peak flu seasons. This scalability is crucial for mass vaccination programs, ensuring that sufficient doses are available to protect at-risk populations.
Ongoing research and development efforts focus on enhancing the efficacy of IIVs. Innovations include advancements in adjuvant technology to boost immune responses and efforts to create more strain-specific vaccines that better match circulating viruses. Increased government initiatives promoting flu vaccination contribute to the growth of the IIV segment. Public health campaigns aimed at raising awareness about vaccination benefits further drive demand.
Furthermore, key players in the industry more focus on research activities and the rising number of clinical trials that would propel this segment's growth in the influenza vaccines market. For instance, in September 2024, GSK announced positive headline data from its Phase II trial of a seasonal influenza mRNA vaccine program, indicating promising results for its vaccine candidates against both influenza A and B strains. This trial involved testing various mRNA formulations in healthy adults across two age groups: younger adults (ages 18 to 64) and older adults (ages 65 to 85).
Also, in September 2023, the National Institutes of Health (NIH) launched a Phase 1 clinical trial for an experimental universal influenza vaccine, aiming to provide broad protection against multiple strains of the influenza virus. This initiative is part of ongoing efforts to develop a vaccine that could potentially eliminate the need for annual flu shots and improve public health responses to seasonal and pandemic influenza outbreaks. These factors have solidified the segment's position in the global influenza vaccine market.
Influenza Vaccine Market Geographical Share
North America is expected to hold a significant position in the global influenza vaccine market share
North America holds a substantial position in the global influenza vaccine market and is expected to hold most of the market share.
The prevalence of seasonal influenza in North America is a major driver of vaccine demand. The Centers for Disease Control and Prevention (CDC) reports that there are approximately 3 to 5 million cases of severe influenza globally each year, with a substantial portion occurring in the U.S. This high incidence rate underscores the necessity for effective vaccination strategies to protect public health.
North America boasts a well-established healthcare system that facilitates efficient vaccine distribution and administration. This infrastructure includes advanced healthcare facilities, a robust network of pharmacies, and widespread access to immunization services, ensuring that vaccines are readily available to the population. Strong government initiatives and funding for vaccination programs significantly contribute to market growth. Public health campaigns aimed at increasing awareness about the importance of flu vaccinations help drive demand, particularly among high-risk groups such as the elderly and healthcare workers.
Innovations in vaccine production technology enhance the efficacy and safety of influenza vaccines. The development of quadrivalent vaccines, which protect against four strains of the virus, is particularly noteworthy. Additionally, advancements in digital vaccination monitoring and tracking systems improve vaccine distribution efficiency. The North American market is home to several leading pharmaceutical companies that invest heavily in research and development for influenza vaccines. This competitive landscape fosters rapid product launches and continuous innovation, contributing to the overall growth of the market.
Furthermore, key players in the industry more focus on research activities and the rising number of clinical trials that would propel this segment's growth in the influenza vaccines market in the region. For instance, in June 2024, Moderna, Inc. announced that its Phase 3 trial for the investigational combination vaccine mRNA-1083, which targets both influenza and COVID-19, has successfully met its primary endpoints. The trial demonstrated that this combination vaccine elicited a higher immune response compared to the licensed vaccines currently used for these diseases.
Thus, the above factors are consolidating the region's position as a dominant force in the global influenza vaccine market.
Asia Pacific is growing at the fastest pace in the global influenza vaccine market share
Asia Pacific holds the fastest pace in the global influenza vaccine market and is expected to hold most of the market share.
The increasing prevalence of seasonal influenza outbreaks across Asia-Pacific countries, particularly in densely populated nations like China and India, significantly fuels demand for vaccines. Reports indicate that influenza affects millions annually, underscoring the urgent need for effective vaccination strategies to protect public health. Strong government initiatives aimed at promoting vaccination against influenza play a crucial role in market growth. Many countries in the region are enhancing their public health campaigns to raise awareness about the importance of flu vaccinations, especially among high-risk populations such as children, the elderly, and healthcare workers.
There is a notable increase in investments in healthcare infrastructure across Asia-Pacific, facilitating better vaccine distribution and administration. Improved healthcare facilities and expanded access to immunization services ensure that vaccines are readily available to the population. Innovations in vaccine production technology, including the development of quadrivalent vaccines that protect against four strains of the virus, enhance the efficacy and safety of influenza vaccines. Additionally, advancements in digital health technologies improve vaccine tracking and monitoring systems, contributing to more efficient vaccination campaigns.
The focus on emerging economies within the Asia-Pacific region is driving market growth. Countries like India and South Korea are witnessing increased demand for influenza vaccines due to their large patient pools and rising healthcare infrastructure. These markets benefit from less stringent regulations that facilitate quicker vaccine development and distribution.
Furthermore, key players in the industry product launches that would drive this influenza vaccine market growth. For instance, in March 2024, Cadila Pharmaceuticals announced the launch of its new Cadiflu Tetra vaccine, designed to prevent influenza, a widespread viral infection. This quadrivalent vaccine has received approval from the Drugs Controller General of India (DCGI) for use in both adults and children.
Also, in October 2023, Mylab and the Serum Institute of India (SII) launched Nasovac S4, India's first needle-free nasal influenza vaccine. This innovative vaccine is designed to protect against influenza through a convenient intranasal administration method, making it more accessible and less intimidating, especially for children and individuals with a fear of needles.
Thus, the above factors are consolidating the region's position as the fastest-growing force in the global influenza vaccine market.
Influenza Vaccine Market Major Players
The major global players in the influenza vaccine market include Sanofi, GSK plc., Pfizer Inc., AstraZeneca, CSL, Viatris Inc. (Mylan), Bharat Biotech., SINOVAC, Zydus Cadila Healthcare Limited, and Serum Institute of India Pvt. Ltd. among others.
Key Developments
- In September 2024, the FDA approved FluMist, a nasal spray influenza vaccine, for self- or caregiver administration, marking it as the first flu vaccine that does not require administration by a healthcare professional. This approval represents a significant advancement in making influenza vaccination more accessible and convenient for individuals and families.
| Metrics | Details | |
| CAGR | 6.5% | |
| Market Size Available for Years | 2022-2032 | |
| Estimation Forecast Period | 2025-2032 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Product Type | Inactivated, Live Attenuated |
| Category | Quadrivalent, Trivalent | |
| Route of Administration | Injection, Nasal Spray | |
| Age Group | Pediatric, Adults | |
| End-User | Hospitals, Specialty Clinics, Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials, and product pipelines, and forecasts upcoming advancements in medical devices and pharmaceuticals.
- Product Performance & Market Positioning: Analyzes product performance, market positioning, and growth potential to optimize strategies.
- Real-world Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: Covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyzes competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient product delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global influenza vaccine market report delivers a detailed analysis with 60+ key tables, more than 50 visually impactful figures, and 176 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2025
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.