Gene Therapy for Rare Disease Market Size
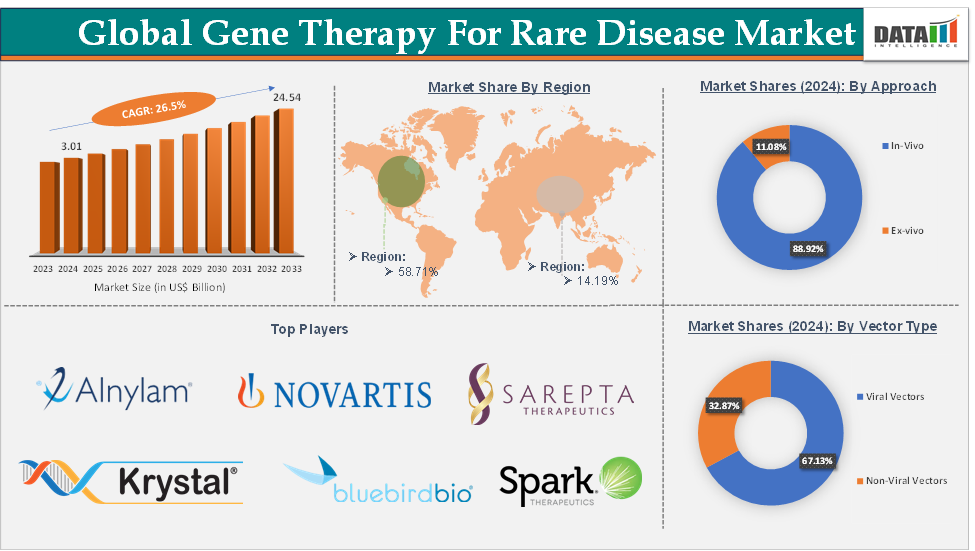
Gene Therapy for Rare Disease Market Size reached US$ 3.01 Billion in 2024 and is expected to reach US$ 24.54 Billion by 2033, growing at a CAGR of 26.5% during the forecast period 2025-2033.
Gene Therapy for Rare Disease Market Overview
The gene therapy market for rare diseases is poised for continued growth, driven by ongoing technological innovations, expanding clinical applications, and increased investment. However, addressing the challenges related to cost, access, and regulatory complexities will be critical for realizing the full potential of gene therapies. Collaborative efforts between governments, pharmaceutical companies, and non-profits will be essential to ensure that gene therapies can be available to needy patients, especially in underserved regions.
Expansion into emerging markets is further accelerating the market growth. For instance, in May 2025, Sarepta Therapeutics, Inc. announced that the Japanese Ministry of Health, Labour, and Welfare (MHLW) approved ELEVIDYS (delandistrogene moxeparvovec) for the treatment of Duchenne muscular dystrophy (DMD) under the conditional and time-limited approval pathway in Japan. ELEVIDYS is approved for individuals aged 3 to less than 8 years old, who do not have any deletions in exon 8 and/or exon 9 in the DMD gene, and who are negative for anti-AAVrh74 antibodies. This is the first global approval to include individuals younger than 4 years of age.
Executive Summary
For more details on this report – Request for Sample
Gene Therapy for Rare Disease Market Dynamics: Drivers & Restraints
Advancements in gene editing technologies are significantly driving the gene therapy for rare disease market growth
CRISPR-Cas9, a revolutionary gene-editing tool, allows scientists to make highly targeted alterations in the DNA sequence. This technology has unlocked new possibilities for treating rare diseases by directly correcting genetic mutations at their source, offering a more effective solution compared to traditional therapies. The approval of Exagamglogene autotemcel (Casgevy), a CRISPR-based gene therapy for Sickle Cell Disease (SCD), marks a significant milestone.
For instance, in January 2024, Vertex Pharmaceuticals Incorporated released that the Saudi Food and Drug Authority (SFDA) granted Marketing Authorization for CASGEVY (exagamglogene autotemcel [exa-cel]), a CRISPR/Cas9 gene-edited therapy, for the treatment of sickle cell disease (SCD) and transfusion-dependent beta-thalassemia (TDT). CASGEVY is approved for treating people 12 years of age and older with SCD or TDT. It demonstrates how CRISPR is used to provide a potentially curative solution for rare genetic conditions
Gene editing allows for more personalized approaches, such as delivering treatments to specific tissues or organs affected by rare diseases. This increases the therapy's effectiveness while minimizing unwanted side effects. For instance, for Inherited Retinal Diseases (IRD), gene editing tools are used to correct genetic mutations in retinal cells, potentially restoring vision. Luxturna is a prime example of gene therapy targeting inherited retinal conditions, and advancements in editing technologies could extend this to rarer diseases.
The high cost associated with gene therapies is hampering the gene therapy for rare disease market.
Gene therapies often involve complex manufacturing processes, such as the production of viral vectors (used to deliver the therapeutic genes), which require highly specialized facilities and technology. This makes the production process expensive. For instance, the gene therapies Zynteglo and Skysona are priced at US$ 2.8 million and US$ 3 million per dose. Moreover, Zolgensma has a reported list price of US$ 2.1 million. This price tag is due to the intricate manufacturing process, including the creation of the adeno-associated virus (AAV) vector used in the therapy.
The development of gene therapies involves lengthy and expensive clinical trials, especially when targeting rare diseases with small patient populations. These trials require extensive regulatory approvals, advanced scientific research, and personalized patient treatments. For instance, Luxturna, a gene therapy for Leber’s congenital amaurosis, took over 10 years from development to approval, and its cost is $850,000 per patient.
Gene Therapy for Rare Disease Market Segment Analysis
The global gene therapy for rare disease market is segmented based on approach, vector type, technique, application, and region.
Vector Type:
The viral vectors segment is expected to hold 67.1% of the market share in 2024 in the gene therapy for rare disease market
Viral vectors, especially Adeno-Associated Viruses (AAVs), are highly efficient at delivering therapeutic genes into target cells. They can transduce a wide range of cell types and tissues, including those in the liver, muscles, and eyes, which is essential for treating rare genetic diseases that affect these organs. For instance, Zolgensma, a gene therapy for Spinal Muscular Atrophy (SMA), uses an AAV vector to deliver a functional copy of the SMN1 gene to motor neurons. This treatment has been successful in providing long-term benefits to young patients and highlights the importance of viral vectors in treating neuromuscular diseases.
Despite some concerns about immune responses, viral vectors have been extensively studied and have demonstrated strong safety profiles in clinical trials. Their ability to integrate genetic material into patient cells with minimal risk of off-target effects is a significant factor in their dominance. For instance, Luxturna, a gene therapy for inherited retinal diseases, uses an AAV vector to deliver a corrected version of the RPE65 gene directly to retinal cells. It has shown significant improvements in vision for patients, further proving the safety and effectiveness of viral vectors for treating genetic diseases.
Gene Therapy for Rare Disease Market, Geographical Analysis
North America is expected to dominate the global gene therapy for rare disease market with a 58.7% share in 2024
North America, especially the US FDA, offers Orphan Drug Designation and fast-track approval processes for gene therapies targeting rare diseases. This regulatory support accelerates the development and commercialization of gene therapies in North America, making it a key region for the adoption of these treatments. The list of some gene therapies approved by the FDA includes:
Gene Therapy | Indication |
CASGEVY and LYFGENIA | Sickle Cell Disease |
ELEVIDYS | Duchenne Muscular Dystrophy |
KYMRIAH | Leukemia and Lymphoma |
LUXTURNA | Inherited Retinal Disorder |
ROCTAVIAN | Hemophilia A |
SKYSONA | Cerebral Adrenoleukodystrophy (CALD) |
ZOLGENSMA | Spinal Muscular Atrophy (SMA) |
ZYNTEGLO and CASGEVY | Beta Thalassemia |
Many leading biotech companies headquartered in North America are at the forefront of gene therapy development. These companies play a critical role in pushing forward research, clinical trials, and product commercialization, making North America the global leader in gene therapy advancements. For instance, Spark Therapeutics, based in the U.S., developed Luxturna, the first FDA-approved gene therapy for inherited retinal disease. This success story highlights North America's leadership in gene therapy innovation and treatment for rare diseases.
Gene Therapy for Rare Disease Market Top Companies
Top companies in the gene therapy for rare disease market include Alnylam Pharmaceuticals, Inc., Spark Therapeutics, Inc., Novartis AG, bluebird bio, Inc., Ferring Pharmaceuticals Inc., Vertex Pharmaceuticals Incorporated, Sarepta Therapeutics, Inc., CSL Behring LLC, Amgen, Inc., Orchard Therapeutics Group., Krystal Biotech, Inc., and others.
Market Scope
Metrics | Details | |
CAGR | 26.5% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Approach | Ex-Vivo and In-Vivo |
Vector Type | Viral Vectors and Non-Viral Vectors | |
Technique | Gene Addition, Gene Silencing, and Gene Editing | |
Application | Oncology, Musculoskeletal Conditions, Blood Disorders, Ophthalmology, and Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global gene therapy for rare disease market report delivers a detailed analysis with 73 key tables, more than 63 visually impactful figures, and 168 pages of expert insights, providing a complete view of the market landscape.