AI in Clinical Trials Market Size & Industry Outlook
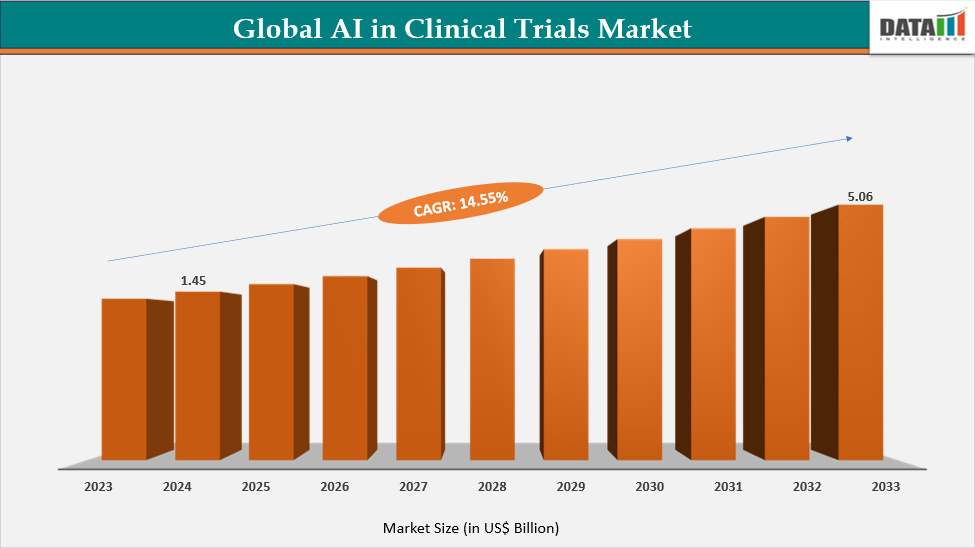
The global AI in Clinical Trials market reached US$1.45 billion in 2024, up from US$1.27 billion in 2023, and is projected to reach US$5.06 billion by 2033, growing at a robust CAGR of 14.55% from 2025 to 2033. The market’s rapid expansion is primarily driven by the rising adoption of artificial intelligence technologies to enhance clinical trial efficiency, reduce costs, and accelerate drug development timelines. Increasing data availability from electronic health records (EHRs), genomics, and real-world evidence is enabling AI algorithms to optimize patient recruitment, predict trial outcomes, and improve protocol design.
Moreover, the growing prevalence of complex diseases such as cancer and neurological disorders has prompted pharmaceutical and biotechnology companies to leverage AI-powered analytics for precision trial management and adaptive study designs. Additionally, the growing collaboration between AI technology providers, contract research organizations (CROs), and life science companies is further fueling innovation in this space. As regulatory bodies continue to embrace digital and data-driven methodologies, the global AI in Clinical Trials market is expected to witness sustained growth, offering transformative potential for accelerating medical breakthroughs and improving patient outcomes.
Key Market Highlights
- North America dominates the AI in Clinical Trials market with the largest revenue share of 43.04% in 2024.
- North America is the fastest-growing region and is expected to grow at the fastest CAGR of 17.18% over the forecast period.
- Based on the offering type, the software segment led the market with the largest revenue share of 67.43% in 2024.
- The major market players in the AI in Clinical Trials market are Medidata, IQVIA, Saama Technologies, Phesi, Euretos, Median Technologies, Innoplexus, Deep6.ai, AiCure, Antidote Technologies, Mendel AI, among others.
Market Dynamics
Drivers: The Rising R&D Costs and Long Trial Timelines are significantly driving the AI in Clinical Trials market growth
The rising R&D costs and extended timelines associated with traditional clinical trials are among the most significant drivers of the AI in Clinical Trials market. Pharmaceutical and biotechnology companies are increasingly seeking solutions that can accelerate patient recruitment, streamline data analysis, and enhance trial efficiency. AI technologies fit this demand perfectly, offering benefits such as predictive patient enrollment, automated monitoring, adaptive trial design, and real-time data analytics, which help reduce costs and shorten timelines.
According to the U.S. Food and Drug Administration (FDA), it takes, on average, 10 to 15 years and approximately $2.6 billion to bring a new medicine to market. This lengthy and costly process underscores the need for innovative solutions to expedite drug development and reduce expenses.
In addition, the European Medicines Agency (EMA) has recognized the potential of AI in clinical trials. The agency's human medicines committee (CHMP) has accepted clinical trial evidence generated by an AI tool supervised by a human pathologist, marking a significant step in integrating AI into clinical research.
Furthermore, the FDA has highlighted the role of AI in mitigating existing challenges in conducting post-market surveillance for generic drugs, including insufficient data on bioequivalence and high costs associated with large-scale studies. AI and machine learning (ML) have the potential to address these challenges by improving data analysis and decision-making processes.
Leading technology providers and Contract Research Organizations (CROs), including IBM Watson Health, Medidata, and IQVIA, are expanding AI-powered offerings to optimize trial design and patient outcomes. The adoption of AI-driven predictive analytics and real-world data integration is making clinical trials more efficient and precise, paving the way for faster drug development and improved patient access to innovative therapies.
Restraints: Shortage of Skilled Professionals is hampering the growth of the market
The shortage of skilled professionals in clinical research is a significant restraint on the growth of the AI in Clinical Trials market. Pharmaceutical companies and Contract Research Organizations (CROs) are increasingly adopting AI technologies to enhance trial efficiency and data analysis. However, the rapid pace of AI integration has outstripped the availability of professionals with the requisite expertise in both AI and clinical development. This talent gap hampers the effective implementation and scaling of AI solutions in clinical trials.
A 2025 report by Intuition Labs highlights that 49% of surveyed industry professionals identified a shortage of specific skills and talent as the primary barrier to their company's digital transformation. This shortage is particularly pronounced in roles requiring proficiency in machine learning, data science, and regulatory compliance within the context of clinical research.
This talent shortage not only affects the deployment of AI technologies but also poses risks to the quality and compliance of clinical trials, potentially leading to increased operational costs and delayed time-to-market for new therapies.
For more details on this report – Request for Sample
AI in Clinical Trials Market, Segment Analysis
The global AI in Clinical Trials market is segmented based on offering, trial phase, indication, end user, and region.
Offering: The software segment is dominating the AI in Clinical Trials market with a 67.43% share in 2024
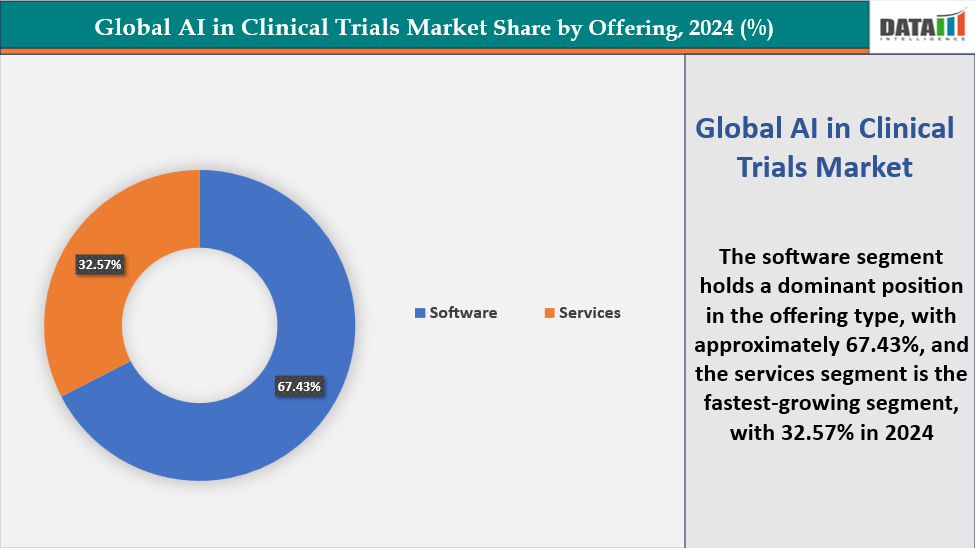
The software segment is dominating the AI in Clinical Trials market, accounting for a 67.43% share in 2024. Software solutions, including clinical trial management systems (CTMS), electronic data capture (EDC) platforms, and AI-driven analytics tools, are at the forefront of digitizing and optimizing clinical trials. These platforms enable streamlined protocol design, real-time patient monitoring, predictive enrollment, and automated data analysis, significantly reducing trial costs and timelines.
Pharmaceutical companies and Contract Research Organizations (CROs) increasingly rely on AI software to handle the growing complexity of clinical trial data, particularly with the rise of multi-center and global trials. For example, Medidata Solutions’ AI-enabled Rave platform has been widely adopted to manage large-scale clinical trials, providing predictive insights and real-time reporting that enhance decision-making. Similarly, IBM Watson Health offers AI-driven clinical trial matching and analytics software that accelerates patient recruitment and optimizes trial outcomes.
The dominance of the software segment is further reinforced by the scalability and flexibility of cloud-based solutions, which allow seamless integration with existing trial workflows and support remote or decentralized trials. As clinical trials become more data-intensive and adaptive, AI software continues to be the key driver for improving efficiency, reducing human errors, and enabling faster, more precise drug development.
Geographical Analysis
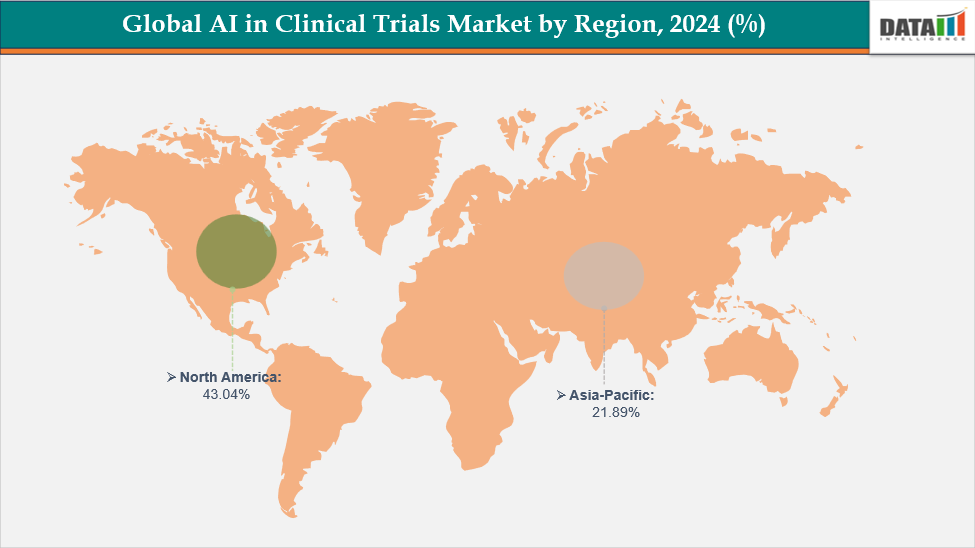
North America dominates the global AI in Clinical Trials market with a 43.04% in 2024
North America dominates the global AI in Clinical Trials market, accounting for 43.04% of the market share in 2024. The region’s leadership is driven by the presence of a robust pharmaceutical and biotechnology industry, advanced healthcare infrastructure, and early adoption of AI technologies in clinical research. The U.S., in particular, is home to major AI technology providers, Contract Research Organizations (CROs), and leading pharmaceutical companies that are actively implementing AI-driven solutions for patient recruitment, trial monitoring, and data analytics.
Government support and regulatory frameworks also play a key role in accelerating AI adoption. Agencies such as the U.S. Food and Drug Administration (FDA) have issued guidance and pilot programs for the integration of AI and machine learning in clinical trials, enabling companies to safely leverage AI for trial design and real-world data analysis. Additionally, the widespread availability of electronic health records (EHRs), large patient databases, and real-world data in the region facilitates AI-driven insights and predictive modeling, further strengthening North America’s position.
Leading players, such as IBM Watson Health, Medidata Solutions, and IQVIA, are headquartered or heavily active in North America, contributing significantly to technological innovation and service offerings. The region’s strong focus on precision medicine, digital health initiatives, and decentralized clinical trials continues to reinforce its dominance in the global AI in Clinical Trials market.
Europe AI in Clinical Trials Market Trends
In the European region, the AI in Clinical Trials market is witnessing steady growth, driven by several key factors. The European Union has launched a €1.1 billion initiative, the "Apply AI" strategy, to accelerate AI deployment across critical industries, including healthcare. This initiative aims to reduce dependence on non-EU technologies while fostering innovation within the region. Regulatory support is further strengthened by the European Medicines Agency (EMA), which has approved AI tools such as AIM-NASH for assessing liver disease severity, reflecting growing confidence in AI’s application in clinical trials. Additionally, AI-driven platforms are being increasingly adopted to optimize trial design, streamline patient recruitment, and enable real-time monitoring, significantly enhancing trial efficiency and data accuracy.
With strong regulatory backing, technological advancements, and strategic investments, Europe is poised to continue leading the integration of AI in clinical trials, improving the speed, precision, and cost-effectiveness of drug development across the region.
Asia Pacific AI in Clinical Trials Market Trends
In the Asia Pacific region, the AI in Clinical Trials market is experiencing rapid growth, driven by several key factors. Increasing R&D investments in countries like China and India are creating a conducive environment for clinical trials and AI adoption. The region’s high burden of chronic diseases, including diabetes, cardiovascular conditions, and cancer, is fueling the need for efficient and innovative trial solutions. Additionally, the large and diverse patient population in the Asia Pacific provides a rich pool for inclusive and representative clinical studies. Cost-effectiveness is another critical driver, as conducting trials in this region is more economical compared to Western countries.
Government support through favorable policies and investment in healthcare infrastructure is further promoting the integration of AI technologies. Technological advancements, such as cloud computing and sophisticated AI platforms, are enabling more efficient data management, real-time monitoring, and predictive analytics. Strategic collaborations between pharmaceutical companies, technology providers, and research institutions are accelerating the development and application of AI in clinical trials. Together, these factors position the Asia Pacific region as a rapidly expanding hub for AI-driven clinical research, enhancing trial efficiency, reducing costs, and improving patient outcomes.
AI in Clinical Trials Competitive Landscape
Top companies in the AI in Clinical Trials market include Medidata, IQVIA, Saama Technologies, Phesi, Euretos, Median Technologies, Innoplexus, Deep6.ai, AiCure, Antidote Technologies, Mendel AI, among others.
Market Scope
| Metrics | Details | |
| CAGR | 14.55% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Offering | Software and Services |
| Trial Phase | Phase I, Phase II, and Phase III | |
| Indication | Oncology, Central Nervous System (CNS) Disorders, Cardiovascular (CVS) Diseases, and Other Indications | |
| End-user | Pharmaceutical and Biotechnology Companies, Contract Research Organizations (CROs), Other End Users | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global AI in Clinical Trials market report delivers a detailed analysis with 73 key tables, more than 63 visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more healthcare IT-related reports, please click here