Triple Negative Breast Cancer Treatment Market Size and Trends
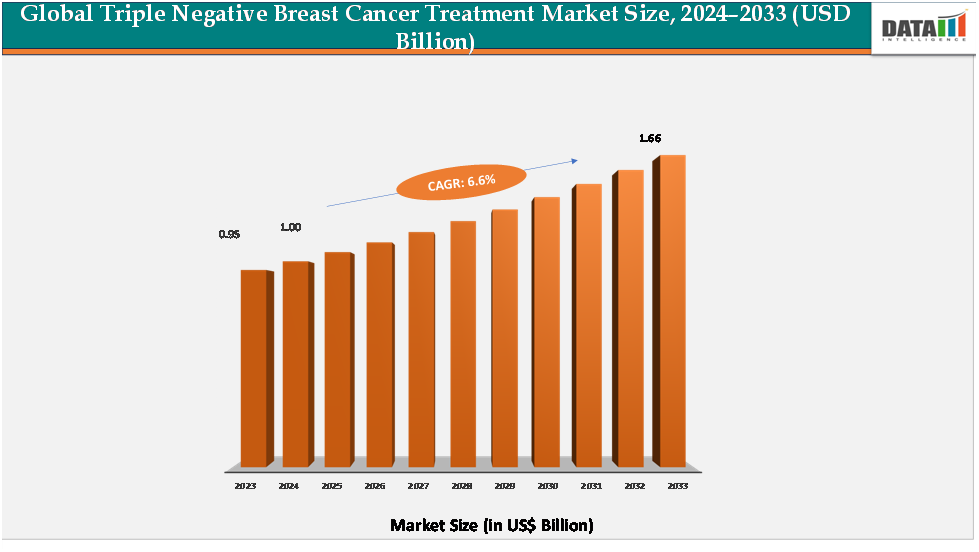
The global triple negative breast cancer Treatment Market reached US$ 0.95billion in 2023, with a rise to US$ 1.00billion in 2024, and is expected to reach US$ 1.66billion by 2033, growing at a CAGR of 6.6% during the forecast period 2025–2033.
The growing focus on precision medicine, targeted therapies, and immunotherapies is transforming the triple negative breast cancer (TNBC) treatment market, as these advanced treatments are becoming essential for improving patient outcomes. By offering personalized, highly effective, and less toxic options compared to conventional chemotherapy, novel therapies are enabling oncologists to address aggressive TNBC subtypes more efficiently while minimizing side effects.
Furthermore, rapid advancements in antibody-drug conjugates (ADCs), immune checkpoint inhibitors, and combination therapies are aligning with global expectations for faster, safer, and more effective cancer care, while the expansion of treatment accessibility across emerging markets and specialized cancer centers is creating new opportunities for wider adoption and long-term market growth.
Key Market highlights
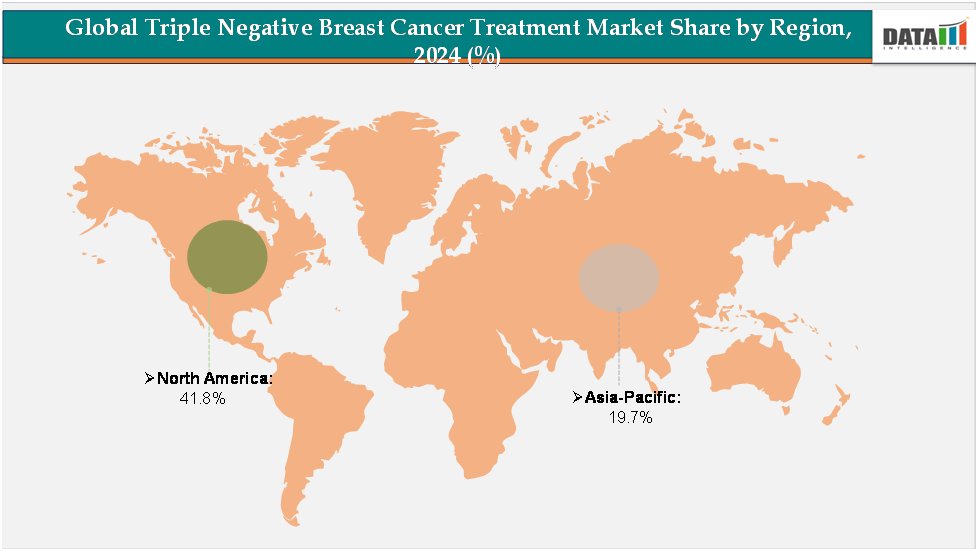
- North America leads the triple negative breast cancer (TNBC) treatment market, accounting for approximately 41.8% of global revenue, supported by its high breast cancer screening rates and strong presence of leading pharmaceutical and biotechnology companies. The region’s dominance is further reinforced by the rapid adoption of novel therapies such as immunotherapy and antibody-drug conjugates, coupled with favorable reimbursement policies and a robust clinical trial pipeline.
- Asia-Pacific represents the fastest-growing regional market, holding about 19.7% of the share, driven by rising breast cancer prevalence in countries such as China, India, Japan, and South Korea. Increasing government investments in oncology care, expansion of specialized cancer centers, and greater patient access to targeted therapies are fueling growth.
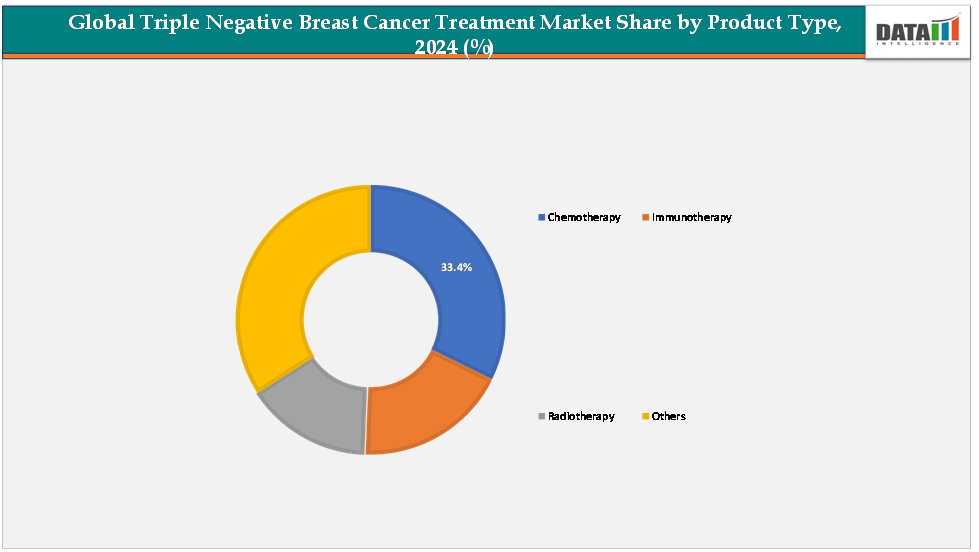
- Chemotherapy remains the dominant treatment segment, contributing around 33.4% of the market revenue. Despite the emergence of targeted therapies and immunotherapies, chemotherapy continues to be the standard first-line treatment due to its widespread availability, established clinical outcomes, and comparatively lower cost.
Market Size & Forecast
- 2024 Market Size: US$1.00Billion
- 2033 Projected Market Size: US$1.66Billion
- CAGR (2025–2033): 6.6%
- North America: Largest market in 2024
Asia Pacific: Fastest-growing market
Source : Datam Intelligence Email : [email protected]
Drivers & Restraints
Driver: Rising Focus on Infection Control in Healthcare Facilities
The rising incidence of triple-negative breast cancer (TNBC) is significantly driving the growth of the TNBC treatment market. Recent data indicate that TNBC accounts for approximately 10–15% of all breast cancer cases, with an estimated 316,950 new cases of invasive breast cancer expected in women in the U.S. in 2025. Notably, the incidence of TNBC is increasing among specific demographics. Furthermore, Black women are diagnosed with TNBC at rates approximately twice as high as those of White, Hispanic, or Asian/Pacific Islander women. This surge in TNBC cases is propelling demand for advanced therapies, including immunotherapies and antibody-drug conjugates, thereby expanding the treatment market.
Restraint: High Initial Investment and Maintenance Costs
The high cost of novel therapies is a significant factor that could restrain the growth of the Triple Negative Breast Cancer (TNBC) treatment market. Advanced treatments such as targeted therapies, immunotherapies, and antibody-drug conjugates (ADCs) often come with substantial price tags, making them less accessible to a large portion of the patient population, especially in developing regions. Many patients may be unable to afford these therapies without substantial insurance coverage or government support, which limits widespread adoption.
For more details on this report, Request for Sample
Segmentation Analysis
The global triple negative breast cancer treatment market is segmented by treatment type, stage, end-user, and region.
Type:
The chemotherapy segment is estimated to have 43.2% of the triple negative breast cancer treatment market share.
Chemotherapy continues to hold the dominant position in the TNBC treatment market, accounting for the largest share of global revenue. This is primarily due to the aggressive nature of TNBC, which lacks estrogen, progesterone, and HER2 receptors, making hormonal or HER2-targeted therapies ineffective for most patients. As a result, chemotherapy remains the backbone of treatment for early-stage, locally advanced, and metastatic TNBC cases. Established regimens such as anthracyclines and taxanes are widely used, supported by extensive clinical data demonstrating improved survival rates and disease management. The dominance of chemotherapy is further reinforced by its availability across hospitals, specialty cancer centers, and emerging markets, making it the most accessible and widely adopted treatment option globally.
The immunotherapy segment is estimated to have 26.4% of the triple negative breast cancer treatment market share.
Immunotherapy is the fastest-growing segment in the TNBC treatment market, driven by recent breakthroughs in immune checkpoint inhibitors (such as PD-1/PD-L1 inhibitors) and combination therapies that enhance anti-tumor immune response. These therapies are particularly promising for patients with advanced or metastatic TNBC, where traditional chemotherapy shows limited efficacy. The growth is supported by an expanding pipeline of clinical trials, regulatory approvals of drugs like atezolizumab in combination with chemotherapy, and increased adoption of biomarker-driven, personalized treatment strategies. Rising awareness among oncologists and patients, coupled with government and private investments in cancer immunotherapy research, is accelerating adoption, positioning immunotherapy as a rapidly expanding segment in the TNBC treatment market.
Geographical Analysis
The North America triple negative breast cancer treatment market was valued at 41.8%market share in 2024
North America, especially the United States, is expected to dominate the global triple-negative breast cancer (TNBC) treatment market. This dominance is driven by the high prevalence of TNBC in the region, advanced healthcare infrastructure, and the presence of leading pharmaceutical companies actively engaged in research and development of innovative therapies. The U.S. healthcare system offers extensive access to cutting-edge treatments and clinical trials, which encourages the rapid adoption of new therapies. Moreover, strong reimbursement policies and government initiatives in the U.S. ensure accessibility and affordability of advanced cancer treatments, further supporting market growth.
Leading pharmaceutical players are introducing new drugs and therapies by collaborating with other key market players. For instance, in June 2025, BioNTech SE and Bristol Myers Squibb announced a global agreement to co-develop and co-commercialize BioNTech’s investigational bispecific antibody, BNT327, for multiple solid tumor types. Under this partnership, both companies will collaborate to accelerate and expand the development of this promising clinical candidate. A global Phase 3 trial evaluating BNT327 in triple negative breast cancer (TNBC) is planned to commence by the end of 2025. These factors collectively reinforce North America’s pivotal role in shaping the future of TNBC treatment.
The Europe triple negative breast cancer treatment market was valued at 21.4% market share in 2024
Europe maintains a significant position in the TNBC treatment market, with Germany leading the region. In 2024, Germany accounted for a substantial share of the European breast cancer therapeutics market, supported by a robust healthcare infrastructure, strong reimbursement policies, and high per capita healthcare expenditure. The European market emphasizes personalized medicine and targeted therapies.
For instance, in 2025, the National Institute for Health and Care Excellence (NICE) in England approved ribociclib, a drug that slows tumor growth by targeting CDK 4 and 6 proteins, highlighting Europe’s commitment to integrating innovative treatments into clinical practice. Additionally, hospital pharmacies dominate the distribution of breast cancer therapeutics in Europe, holding over two-thirds of the market share in 2024, due to the need for specialized administration of intravenous and injectable therapies. Europe’s focus on advanced treatment protocols and personalized care ensures it remains a significant player in the global TNBC market.
The Asia-Pacific triple negative breast cancer treatment market was valued at 19.7% market share in 2024
The Asia-Pacific (APAC) region is emerging as the fastest-growing market for TNBC treatments. The growth is fueled by increasing cancer awareness, rising healthcare investments, and improved access to diagnostic and therapeutic facilities. Countries like India are witnessing a surge in breast cancer cases, which drives the demand for effective treatment options. The TNBC market in India and other APAC countries is expected to witness the highest compound annual growth rate (CAGR) in the coming years due to expanding cancer screening programs, increasing availability of oncology drugs, and government initiatives aimed at improving cancer care accessibility. Pharmaceutical companies are investing heavily in the region to meet this growing demand. For instance, AstraZeneca is constructing a $1.5 billion manufacturing facility in Singapore dedicated to producing antibody-drug conjugates, leveraging Singapore’s strategic location and advanced infrastructure to serve Southeast Asia’s growing market.
Competitive Landscape
The major players in the triple negative breast cancer treatment market include Merck & Co., Inc., Gilead Sciences, Inc., and F. Hoffmann-La Roche Ltd., among others.
Key Developments:
- In May 2025, Gilead Sciences, Inc. (Nasdaq: GILD) announced that its therapy Trodelvy (sacituzumabgovitecan-hziy) in combination with Keytruda (pembrolizumab) demonstrated a 35% reduction in the risk of disease progression or death (HR: 0.65) compared to the standard treatment of Keytruda plus chemotherapy. This result was observed in the first-line treatment of patients with PD-L1–positive (CPS ≥10) metastatic triple-negative breast cancer (TNBC), marking a significant advancement in improving outcomes for this difficult-to-treat patient population.
- In February 2023, Shanghai Junshi Biosciences Co., Ltd. announced the completion of a pre-specified interim analysis for its Phase III TORCHLIGHT Study (NCT04085276). This randomized, double-blind, placebo-controlled, multi-center clinical trial is evaluating toripalimab in combination with albumin-bound paclitaxel in patients newly diagnosed with stage IV or recurrent/metastatic triple-negative breast cancer (TNBC).
Market Scope
| Metrics | Details | |
| CAGR | 19.5% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US $Bn) | |
| Segments Covered | Treatment Type | Chemotherapy, Immunotherapy, Radiotherapy, Others |
| Stage | Early-stage TNBC, Metastatic / Advanced-stage TNBC | |
| End-User | Hospitals, Specialty Cancer Centers, Research Institutes & Academic Centers, Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global triple negative breast cancer treatment market report delivers a detailed analysis with 70 key tables, more than 66 visually impactful figures, and 195 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more pharmaceutical-related reports, please click here