Tracheostomy Tube Market Size & Industry Outlook
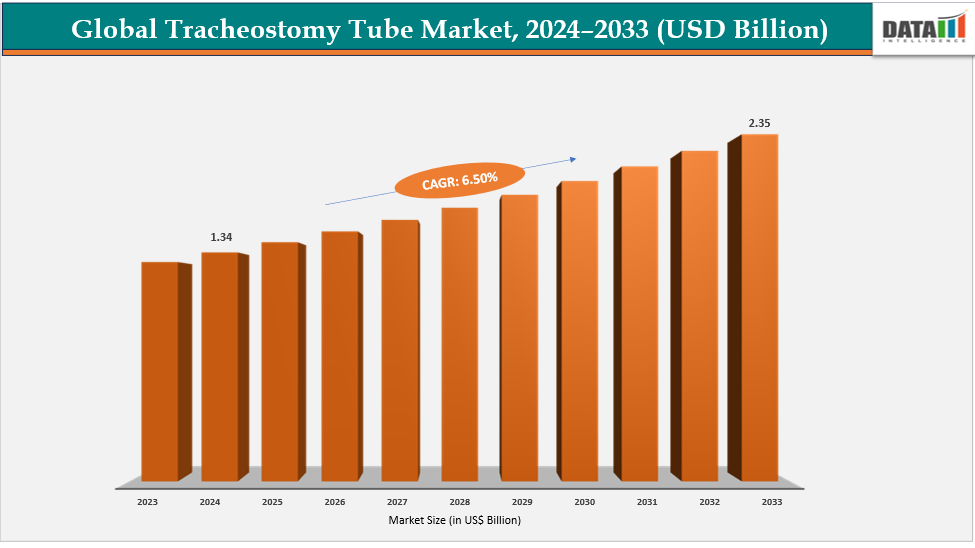
The global tracheostomy tube market size reached US$ 1.26 Billion with a rise of US$ 1.34 Billion in 2024 and is expected to reach US$ 2.35 Billion by 2033, growing at a CAGR of 6.50% during the forecast period 2025-2033.
The global tracheostomy tube market is experiencing steady growth, driven by the rising prevalence of chronic respiratory diseases, an aging population, and advancements in medical technology. The market for tracheostomy tubes is being driven mostly by the expansion of minimally invasive tracheostomy (MIT) procedures. MIT provides smaller incisions, which improves patient safety and physician preference by lowering bleeding, infection, and scarring. Higher procedure numbers are encouraged by shorter intensive care unit stays and quicker recovery, which also reduce hospital expenses. These methods increase the demand for tracheostomy tubes by extending eligibility to high-risk patients, such as the elderly or those with comorbidities. The market is growing as a result of the increased prevalence of chronic respiratory conditions and ventilator dependence. Because of MIT has superior clinical results and cost effectiveness, hospitals and payers prefer it, which encourages the use of sophisticated tubes such cuffed, fenestrated, and inner-cannula designs.
Key Highlights
- North America dominates the tracheostomy tube market with the largest revenue share of 44.5% in 2024.
- The Asia Pacific is the fastest-growing region and is expected to grow at the fastest CAGR of 7.25% over the forecast period.
- Based on type, the cuffed tracheostomy tube segment led the market with the largest revenue share of 33.3% in 2024.
- Top companies in the tracheostomy tube market include Teleflex Incorporated, Medtronic, ICU Medical, Inc., Cook, Medline Industries, LP, Healthcare 21 Ltd., Global Medikit Limited, ANGIPLAST PRIVATE LIMITED, Atos Medical, Boston Medical Products Inc., and Vitaltec Corporation, among others.
Market Dynamics
Drivers: Rising prevalence of chronic respiratory diseases are significantly driving the tracheostomy tube market growth
The global rise in chronic respiratory diseases such as COPD, severe asthma, and late-stage lung cancer is a key driver of tracheostomy tube demand. Frequently, these illnesses result in respiratory failure that necessitates continuous ventilation. Tracheostomy is preferred by physicians over prolonged oral intubation when mechanical ventilation lasts more than a week or when upper-airway obstruction arises in order to minimize problems and enhance patient comfort. Consumables and tube replacement are necessary on a regular basis, which results in repeat business. CRDs are particularly prevalent in aging populations, which increases the number of patients. These elements work together to raise ICU admissions, long-term ventilation cases, and accessory requirements, which in turn greatly expands the market for tracheostomy tubes worldwide.
According to the World Health Organization (WHO), over 80 million people are affected by respiratory diseases, and many more are undiagnosed worldwide.
Moreover, even though 81.7 million people in the region are living with a chronic respiratory disease (CRD) and 6.8 million are newly diagnosed each year, CRDs have slipped from policy priorities, leaving millions without the care and attention they need. COPD and asthma account for the majority of CRD cases in the region, and COPD is responsible for 80% of CRD-related deaths. Future projections indicate that COPD cases will rise globally by 23% between 2020 and 2050, with the steepest increases among women and in low- and middle-income countries. Hospitalization and death from asthma also remain high, particularly among young people, despite the availability of effective treatments.
Restraints: Clinical risks & complications are hampering the growth of the tracheostomy tube market
Clinical risks such as infection, bleeding, tracheal injury, cuff leaks, tube blockage, or accidental decannulation discourage tracheostomy use and slow market growth. Clinicians prefer noninvasive ventilation whenever possible because of these consequences, which increase patient morbidity, lengthen ICU stays, and increase hospital expenses. Device malfunctions may lead to regulatory recalls and more stringent quality standards, which would increase manufacturing costs and postpone the release of new products. Manufacturers bear additional testing and compliance costs, while hospitals and insurers encounter greater liability and reimbursement obstacles. Despite an increase in the prevalence of respiratory diseases, the demand for tracheostomy tubes is being held back globally by a combination of safety concerns and cost pressures.
Owing to the factors like Device failures can trigger regulatory recalls. For instance, in 2023, the U.S. FDA announced a Class I recall of Medtronic’s Shiley Adult Flexible Tracheostomy Tubes after reports that the neck flange could detach, causing loss of airway and serious injury. Hospitals had to remove affected inventory, postpone elective tracheostomies, and shift to alternative devices. This not only disrupted supply but also increased clinician caution toward tracheostomy procedures in general.
For more details on this report, see Request for Sample
Segmentation Analysis
The global tracheostomy tube market is segmented based on type, component, material, end user, and region.
Type: The cuffed tracheostomy tubes from type are dominating the tracheostomy tube market with a 33.3% share in 2024
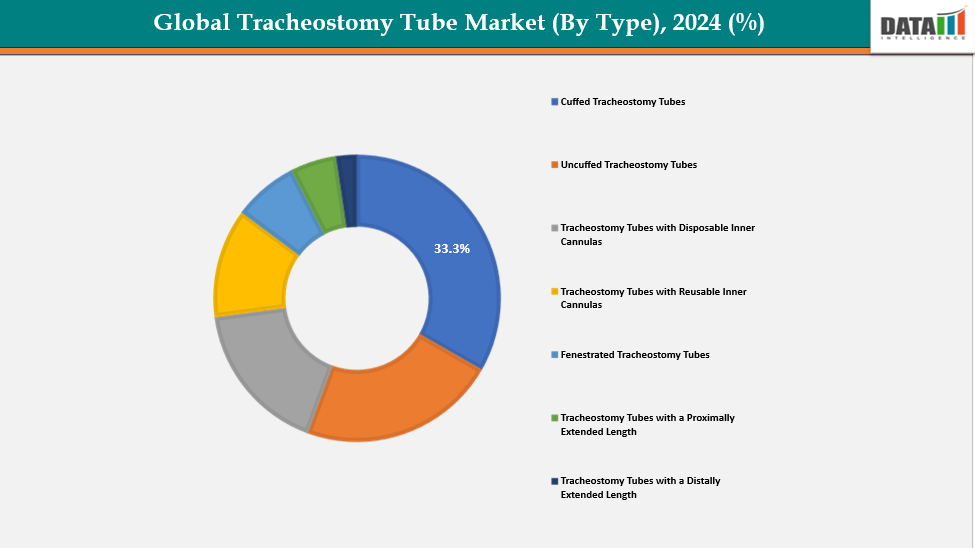
Cuffed tracheostomy tubes dominate the market due to their critical role in adult and ICU care. The inflatable cuff seals the trachea, preventing air leaks and protecting the lungs from aspiration of secretions. Hospitals and surgical centers favor them because they are necessary for critical care treatments, anesthesia, and long-term mechanical ventilation. Improvements in safety, such as low-pressure cuffs, boost acceptance. On the other hand, uncuffed tubes are a minor market segment that is primarily used for pediatric or short-term purposes.
For instance, in June 2025, Vitaltec Corporation received FDA 510(k) clearance for its Rota-Trach Disposable Standard Tracheostomy Tubes, including cuffed models. These tubes are indicated for airway maintenance in tracheostomized patients and are considered substantially equivalent to existing devices.
Material: The polyvinyl chloride (PVC) segment dominates the tracheostomy tube market holds a 42.1% of share in 2024
The Polyvinyl Chloride (PVC) segment dominates the tracheostomy tube market due to its ideal combination of flexibility, strength, and biocompatibility, ensuring patient comfort and safety. Its cost-effectiveness makes it a popular option for hospitals, particularly in emerging nations, and its transparency makes secretion monitoring simple. Additionally, PVC is simple to manufacture in a variety of sizes and shapes, guaranteeing a steady supply and broad availability. Its supremacy is further supported by its long history of clinical acceptance, demonstrated dependability, and compatibility with both cuffed and uncuffed designs. PVC is the most widely used and produced material for tracheostomy tubes due to these factors together.
Geographical Analysis
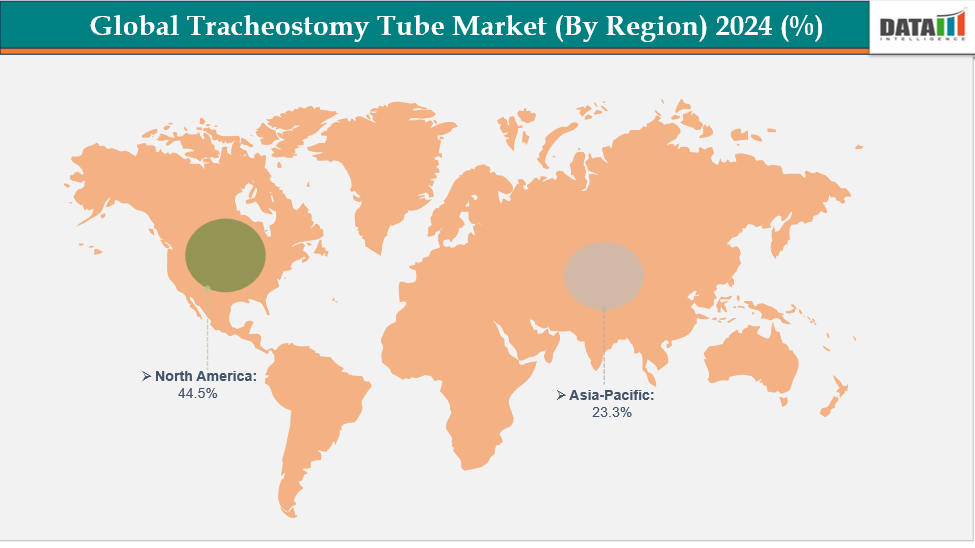
North America dominates the global tracheostomy tube market with 44.5% in 2024.
North America dominates the global tracheostomy tube market due to its advanced healthcare infrastructure, including well-equipped hospitals and ICUs that handle high volumes of tracheostomy procedures. North America, especially the U.S. and Canada, has well-developed hospitals and intensive care units (ICUs). The region has a growing patient population with chronic respiratory diseases, airway obstructions, and age-related ventilation needs, driving demand. High adoption of advanced tube types cuffed, fenestrated, and inner-cannula designs ensures availability of safe and reliable options. Strong R&D, innovation, and regulatory support and approval by the FDA enhance product quality and safety. Additionally, insurance coverage and reimbursement policies make these devices accessible, sustaining North America’s market leadership.
Owing to the factors like regulatory support and approval, in February 2024, Teleflex Medical received FDA 510(k) clearance for the Pilling Tracheostomy Tubes. These tubes featured fenestration for speech, compatibility with speaking valves, durable stainless-steel construction, multiple design styles, reusable single-patient use, and customizable adapters, addressing diverse clinical needs.
Europe is the second region after North America, which is expected to dominate the tracheostomy tube market with 34.5% in 2024.
The European tracheostomy tube market is expected to be the second largest globally, with key contributions from countries such as Germany, the UK, and France. Growth is mostly driven by the region's sophisticated healthcare system, high incidence of chronic respiratory conditions, and rising ICU procedures. Additional factors driving demand include an older population, an increase in injuries and airway blockages, and a stronger focus on early intervention and minimally invasive airway care. Initiatives from groups like the European Respiratory Society (ERS) and supportive regulatory frameworks that improve clinical procedures, improve patient outcomes, and encourage safe, efficient tracheostomy care help to boost Europe's position in the market.
Germany leads the European tracheostomy tube market due to high healthcare spending, advanced hospital and ICU infrastructure, and an aging population. The rising prevalence of chronic respiratory diseases, regulatory approvals, and long-term ventilation needs drives demand, while early adoption of advanced tracheostomy tube types, including cuffed, fenestrated, and inner-cannula designs, accelerates market growth.
Owing to the factors like regulatory approvals, for instance, in April 2024, Atos Medical received CE marking for the Provox LaryTube, a device designed for vocal and pulmonary rehabilitation following total laryngectomy. It serves as a holder for devices in the Provox HME System and is intended for single-patient use.
The Asia Pacific region is the fastest-growing region in the global tracheostomy tube market, with a CAGR of 7.25% in 2024.
The tracheostomy tube market in Asia-Pacific is expanding rapidly, driven by rising cases of chronic respiratory diseases, airway obstructions, and trauma-related ventilation needs. Countries like China, India, Japan, and South Korea are leading this growth due to improved hospital and ICU infrastructure, increasing adoption of advanced tracheostomy tube types, and supportive government healthcare initiatives. Technological advancements, growing awareness of airway management, preference for minimally invasive procedures, and rising investment in respiratory care are further fueling demand across the region, making Asia-Pacific a key focus for market expansion.
The tracheostomy tube market in China is rapidly expanding due to rising ICU admissions, trauma cases, and respiratory complications from urban pollution. Growth is further supported by the expanding private healthcare sector, affordable locally manufactured tubes, and increasing awareness of airway management among clinicians. China’s National Medical Products Administration (NMPA) has strengthened the regulatory framework by issuing new medical device standards and revising existing ones, ensuring patient safety, product quality, and alignment with global norms.
For instance, in February 2024, China’s NMPA updated nine new medical device standards and revised 11 others to enhance the regulatory framework for tracheostomy tubes. These changes aim to improve patient safety, ensure consistent product quality, support advanced tube designs, and align domestic regulations with global standards, facilitating both clinical reliability and international market access.
Competitive Landscape
Top companies in the tracheostomy tube market include Teleflex Incorporated, Medtronic, ICU Medical, Inc., Cook, Medline Industries, LP, Healthcare 21 Ltd., Global Medikit Limited, ANGIPLAST PRIVATE LIMITED, Atos Medical, Boston Medical Products Inc., and Vitaltec Corporation, among others.
Teleflex Incorporated: Teleflex Incorporated, headquartered in Wayne, Pennsylvania, is a global leader in airway management. Its tracheostomy portfolio includes Pilling and Rüsch tubes, offering cuffed, uncuffed, and fenestrated designs for varied clinical needs. The Pilling Tracheostomy Tubes, FDA-cleared in 2024, feature speech-friendly fenestration and durable construction. Teleflex drives market growth through innovation, regulatory compliance, and wide clinical adoption.
Key Developments:
- In July 2024, University Hospitals successfully implanted the first-ever patient-specific 3D-printed T-tube into a human patient. The patient had previously experienced complications with a commercial-grade T-tube. The FDA granted compassionate use for this procedure. The tracheal T-tube, made of silicone and shaped like the letter “T,” allowed the patient to breathe through a neck opening instead of the mouth, providing a customized and safer airway solution.
Market Scope
| Metrics | Details | |
| CAGR | 6.50% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Type | Cuffed Tracheostomy Tubes, Uncuffed Tracheostomy Tubes, Tracheostomy Tubes with Disposable Inner Cannulas, Tracheostomy Tubes with Reusable Inner Cannulas, Fenestrated Tracheostomy Tubes, Tracheostomy Tubes with a Proximally Extended Length, Tracheostomy Tubes with a Distally Extended Length |
| Component | Hub, Flange, Outer Cannula, Inner Cannula, Cuff, Pilot Balloon, Inflation Line | |
| Material | Polyvinyl Chloride, Silicone, Polyurethane and Other | |
| End User | Hospitals, Specialty Clinics, Ambulatory Surgical Centers and Other | |
| Regions Covered | North America, Europe, Asia-Pacific, South America and the Middle East & Africa | |
The Global Tracheostomy Tube Market report delivers a detailed analysis with 73 key tables, more than 73 visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more medical device-related reports, please click here