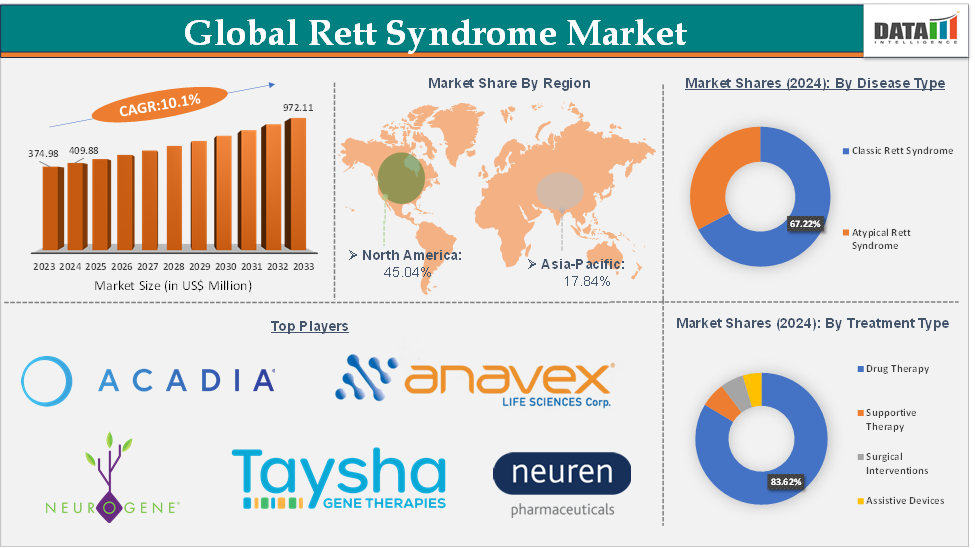
Rett Syndrome Market Size
The global Rett syndrome market size reached US$ 409.88 Million in 2024 from US$ 374.98 Million in 2023 and is expected to reach US$ 972.11 Million by 2033, growing at a CAGR of 10.1% during the forecast period 2025-2033.
Rett Syndrome Market Overview
The Rett Syndrome market represents a promising frontier in rare genetic disease therapeutics, driven by groundbreaking gene therapies and supportive care advancements. While challenges related to cost, patient population size, and regulatory complexity persist, strong unmet needs and technological progress provide a fertile environment for growth. Stakeholders who effectively leverage emerging science, regulatory pathways, and global expansion opportunities are poised to lead this market’s transformation. trategic collaborations, licensing agreements, and partnerships with patient foundations are common to accelerate innovation and market entry.
Rett Syndrome Market Executive Summary
Rett Syndrome Market Dynamics
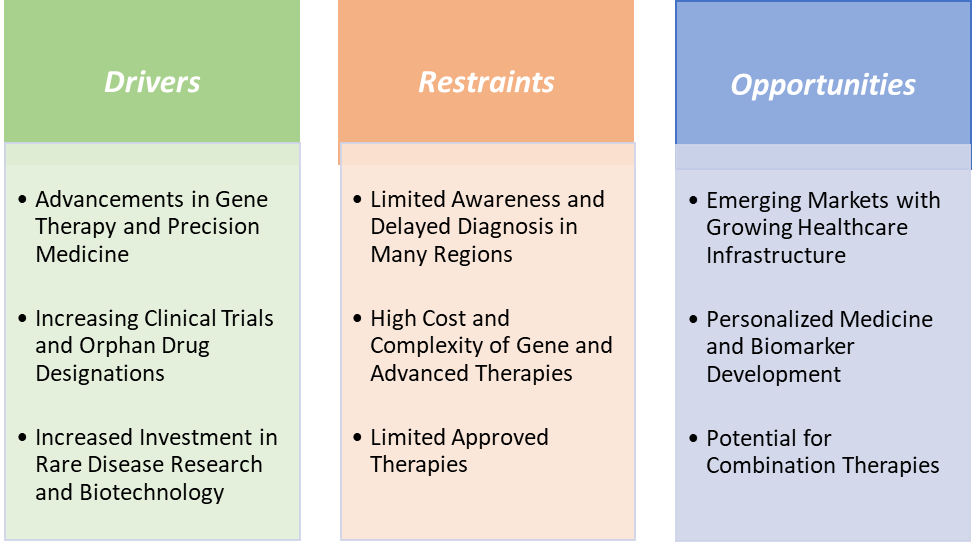
Drivers:
Rett syndrome is primarily caused by mutations in the MECP2 gene, making it an ideal candidate for gene therapy and precision medicine approaches that target the root genetic cause rather than just symptoms. Recent technological breakthroughs and rising clinical trials by market players in gene editing, gene replacement, and RNA-based therapies are transforming the therapeutic landscape. For instance, in June 2024, Neurogene Inc. announced that its NGN-401 gene therapy for Rett syndrome has been selected to participate in the U.S. Food and Drug Administration (FDA) Support for Clinical Trials Advancing Rare Disease Therapeutics (START) Pilot Program.
Additionally, in July 2024, Taysha Gene Therapies, Inc. announced positive longer-term clinical data from the ongoing REVEAL Phase 1/2 adolescent and adult trial and initial clinical data from the REVEAL Phase 1/2 pediatric trial evaluating TSHA-102 in Rett syndrome. TSHA-102 is a self-complementary intrathecally delivered AAV9 investigational gene transfer therapy in clinical evaluation for Rett syndrome.
Advancements in gene therapy and precision medicine are revolutionizing Rett syndrome treatment by addressing the underlying genetic cause. With significant funding, promising clinical trial results, and regulatory incentives, these therapies are poised to capture substantial market share, driving robust growth in an otherwise underserved rare disease market.
Restraints:
Limited awareness and delayed diagnosis in many regions are hampering the growth of the Rett syndrome market
Rett syndrome symptoms often overlap with other neurodevelopmental disorders, leading to misdiagnosis or late diagnosis, sometimes years after symptom onset. In many regions, especially in low- and middle-income countries, healthcare providers lack sufficient knowledge about Rett syndrome, resulting in underreporting and missed opportunities for early intervention. Late or missed diagnosis reduces the number of patients identified and eligible for emerging treatments, directly limiting market size and growth potential.
Limited awareness and delayed diagnosis significantly restrain Rett syndrome market growth by reducing the diagnosed patient population eligible for novel therapies. Improving education, expanding genetic testing access, and awareness campaigns, especially in underserved regions, are critical to unlocking market potential and enabling earlier, more effective treatment.
Opportunities:
Potential for combination therapies creates a market opportunity for the Rett syndrome market
Rett syndrome manifests with a broad spectrum of neurological, motor, respiratory, and cognitive symptoms, which are unlikely to be fully addressed by a single therapy. Combining gene therapies with symptomatic treatments can provide a more comprehensive therapeutic effect. Combination regimens can improve overall quality of life by managing multiple facets of the disease simultaneously, potentially leading to better clinical outcomes and prolonged treatment benefits.
The potential for combination therapies in Rett syndrome offers a significant market opportunity by addressing the disorder’s multifaceted nature. Combining gene therapies with symptomatic and supportive treatments can enhance patient outcomes and expand therapeutic options, thereby creating market opportunity and encouraging innovation in drug development.
Rett Syndrome Market Trends
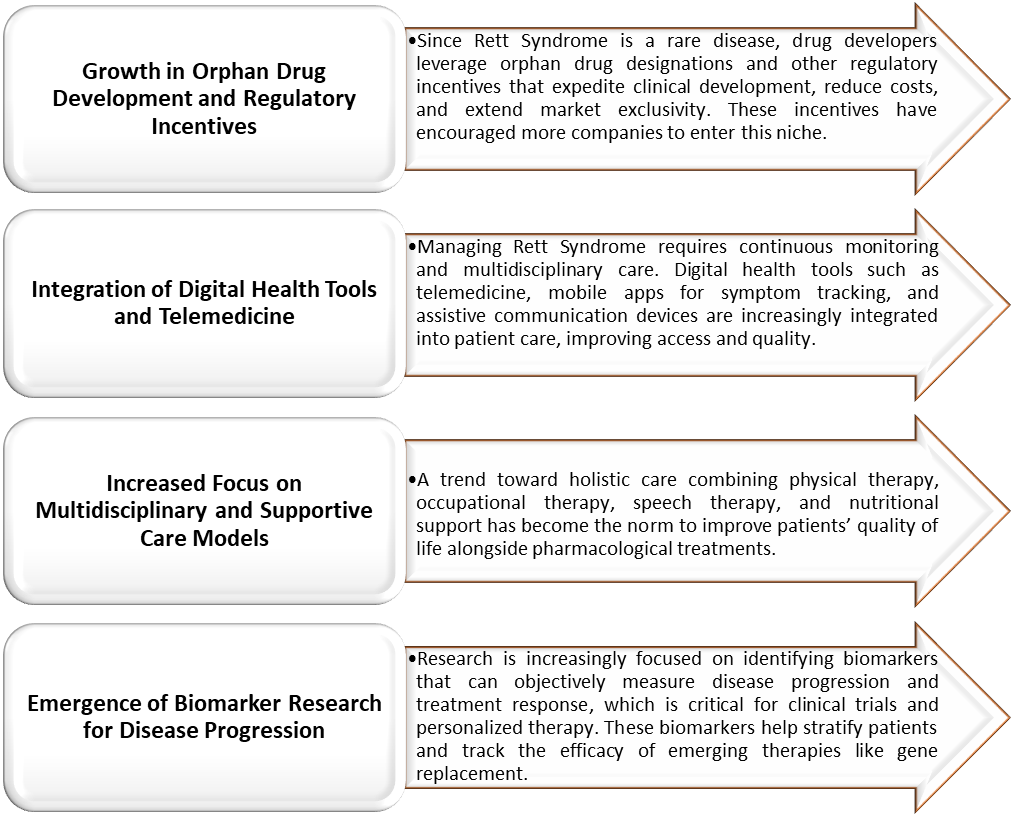
For more details on this report – Request for Sample
Rett Syndrome Market, Segment Analysis
The global Rett syndrome market is segmented based on disease type, treatment type, end-user, and region.
The drug therapy segment from the treatment type is expected to hold 83.62% of the market share in 2024 in the Rett syndrome market
Currently, there is only one approved drug, DAYBUE (trofinetide), for Rett Syndrome. Approved by Acadia Pharmaceuticals Inc. with the U.S. Food and Drug Administration (FDA) approval for the treatment of Rett syndrome in adult and pediatric patients two years of age and older. This approval boosts the sales, which is driving the growth of the drug therapy segment.
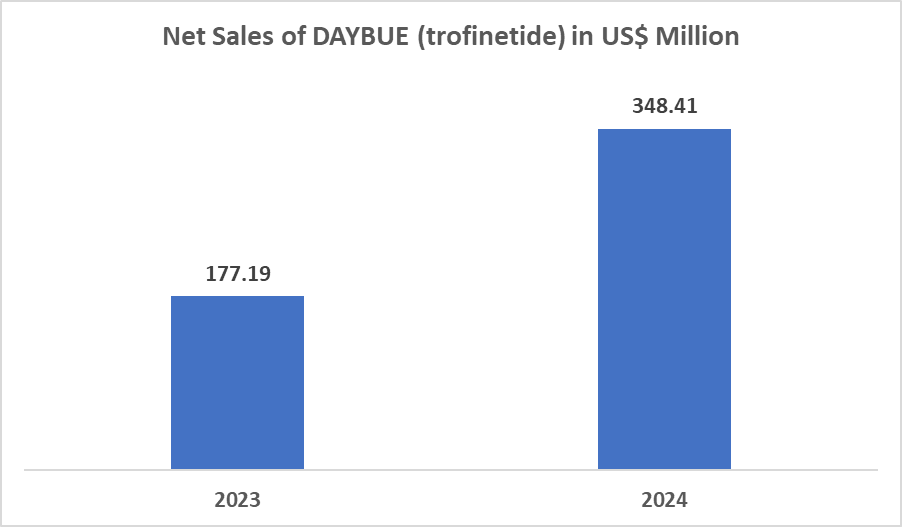
Drug therapies aimed at managing symptoms like seizures, muscle spasticity, and behavioral issues remain the cornerstone of patient care. Numerous pharmaceutical companies are developing both symptomatic drugs and innovative disease-modifying therapies through various clinical trials, fueling market growth.
For instance, in January 2024, Anavex Life Sciences Corp. reported topline results from the randomized, double-blind, placebo-controlled, Phase 2/3 EXCELLENCE clinical trial, which evaluated the clinical efficacy, safety, and tolerability of 30 mg ANAVEX 2-73 in 92 pediatric patients with Rett syndrome (RTT) between the ages of 5 through 17 years, reported positive Real-World Evidence (RWE) feedback from Rett syndrome patients under Compassionate Use Authorization.
Rett Syndrome Market, Geographical Analysis
North America is expected to dominate the global rett syndrome market with a 45.04% share in 2024
Many biotech and pharmaceutical companies developing Rett Syndrome therapies are headquartered or conduct significant clinical research in North America. The U.S. FDA offers orphan drug incentives, fast-track approvals, and breakthrough therapy designations that accelerate Rett syndrome drug development and commercialization. For instance, in March 2023, Acadia Pharmaceuticals Inc. announced that the U.S. Food and Drug Administration (FDA) approved DAYBUE (trofinetide) for the treatment of Rett syndrome in adult and pediatric patients two years of age and older. DAYBUE is the first and only drug approved for the treatment of Rett syndrome.
Over 60% of ongoing rett syndrome clinical trials are conducted in North America, reflecting the region’s research leadership. For instance, Taysha Gene Therapies and Anavex Life Sciences are U.S.-based companies with multiple clinical-stage Rett drug candidates. Genetic testing for Rett Syndrome is more routinely performed in North America, contributing to higher reported prevalence and better patient access to emerging treatments.
Asia-Pacific is growing at the fastest pace in the rett syndrome market, holding 17.84% of the market share
Rapid improvements in healthcare systems, increasing availability of genetic testing, and expanding neurological care centers are enhancing diagnosis rates in countries like China, Japan, South Korea, and India. Patient advocacy groups and government initiatives are boosting awareness about rare diseases, including Rett syndrome, leading to earlier detection and treatment.
Pharmaceutical companies are increasingly investing in rare disease research and clinical trials in the APAC region due to large patient populations and emerging market potential. Rising disposable incomes and improving health insurance coverage enable more families to seek specialized treatments. Governments in countries like China and Japan are adopting faster approval pathways for orphan drugs, attracting global companies.
Rett Syndrome Market Competitive Landscape
Top companies in the rett syndrome market include Acadia Pharmaceuticals Inc., Anavex Life Sciences Corp., AMO Pharma, Numedicus, Neurogene Inc., Taysha Gene Therapies, and Neuren Pharmaceuticals, among others.
Rett Syndrome Market, Key Developments
In May 2025, Neurogene introduced a new monitoring and treatment system for its adeno-associated virus (AAV) gene therapy after a Rett syndrome patient died during a clinical trial. Announced during the American Society of Gene and Cell Therapy (ASGCT) Annual Meeting, Neurogene said the algorithm is intended to reverse the rare, severe hyperinflammatory syndrome haemophagocytic lymphohistiocytosis (HLH), which has been associated with systemic exposure to high doses of AAV gene therapy.
In January 2024, Vanderbilt University Medical Center received a $13 million Department of Defense grant to lead a multisite clinical trial that will evaluate repurposed FDA-approved drugs as treatment options for patients with Rett syndrome.
Rett Syndrome Market Scope
Metrics | Details | |
CAGR | 10.1% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Disease Type | Classic Rett Syndrome and Atypical Rett Syndrome |
Treatment Type | Drug Therapy, Supportive Therapy, Surgical Interventions, and Assistive Devices | |
End-User | Hospitals, Specialty Clinics, Research Institutions & Academic Centers, Homecare Settings, and Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
DMI Insights on Rett Syndrome Market
The Rett syndrome market is witnessing a significant shift from symptomatic treatments to gene therapy and precision medicine approaches targeting the MECP2 gene mutation. Gene replacement and RNA-based therapies are gaining momentum, with several candidates in clinical trials, marking a potential paradigm shift in treatment. This innovation pipeline is expected to reshape the market landscape over the next decade.
Despite progress, challenges like limited awareness, delayed diagnosis, and insufficient genetic testing infrastructure, especially in emerging economies, continue to restrict patient identification and treatment initiation. These factors result in underdiagnosis, limiting the eligible patient pool and restraining market growth.
The market is characterized by the active participation of biotech firms and specialty pharmaceutical companies focusing on rare neurological disorders. Collaborations between industry players, academic institutions, and patient advocacy groups are accelerating innovation and clinical trial recruitment. Awareness campaigns and expanded genetic screening initiatives will gradually improve early diagnosis rates, expanding the market patient base.
The global Rett syndrome market report delivers a detailed analysis with 53 key tables, more than 51 visually impactful figures, and 135 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more pharmaceuticals-related reports, please click here