Disease-Modifying Therapies Market Size & Industry Outlook
The market for disease-modifying therapies (DMTs) is expanding rapidly as a result of developments in small molecules, personalized medicine, and biologics. Compared to traditional medicines, novel biologics such as fusion proteins and monoclonal antibodies offer more effective targeted therapeutic choices that delay the progression of disease. Oral medicines enhance patient adherence and quality of life by offering practical substitutes for injections or infusions. Treatments can be customized to a patient's genetic or biomarker profile due to personalized medicine, which increases effectiveness and lowers negative effects. These cutting-edge treatments have increased therapy options for a variety of diseases and drawn a sizable investment in research and development.
Key Highlights
- North America is dominating the global disease-modifying therapies market with the largest revenue share of 48.5% in 2024.
- The Asia Pacific region is the fastest-growing region in the global disease-modifying therapies market, with a CAGR of 7.7% in 2024.
- The biologics segment is dominating the disease-modifying therapies market with a 60.3% share in 2024
- The multiple sclerosis segment is dominating the disease-modifying therapies market with a 40.3% share in 2024
- Top companies in the disease-modifying therapies market include Genentech USA, Inc.; Novartis AG; Teva Neuroscience, Inc.; GSK; AstraZeneca; Johnson & Johnson; AbbVie Inc.; Amgen Inc.; UCB, Inc.; and Takeda Pharmaceuticals U.S.A., Inc., among others.
Market Dynamics
Drivers: Rising disease prevalence and earlier diagnosis are accelerating the growth of the disease-modifying therapies market
The market for disease-modifying therapies (DMT) is expanding due to increased disease prevalence and earlier diagnosis. More people are being tested for diseases like multiple sclerosis, rheumatoid arthritis, lupus, and Crohn's disease as a result of increased patient and physician awareness. Owing to the factors like prevalence, according to WHO, in 2023, it is estimated that over 1.8 million people have multiple sclerosis worldwide.
Furthermore, early detection often before serious symptoms manifest is made possible by sophisticated diagnostic technologies like MRI, biomarkers, and genetic testing. Timely DMT initiation is made possible by early diagnosis, which enhances patient quality of life and long-term results.
Safety concerns, long‐term efficacy, and adherence issues are hampering the growth of the disease-modifying therapies market
Long-term efficacy issues, safety concerns, and difficulties with adherence are impeding the market expansion for disease-modifying treatments (DMT). Infections, liver damage, and progressive multifocal leukoencephalopathy are among the dangers associated with many DMTs, particularly biologics and immunomodulators, which makes doctors cautious when prescribing them. Infusions and other complex delivery methods, like as injections, enhance the need for monitoring and decrease patient convenience.
Even while diseases including lupus, rheumatoid arthritis, and multiple sclerosis are becoming more common, these variables work together to impede patient acceptance, decrease treatment persistence, and raise healthcare costs, which prevents broader adoption.
For more details on this report, Request for Sample
Disease-Modifying Therapies Market, Segment Analysis
The global disease-modifying therapies market is segmented based on drug class, disease indication, route of administration, distribution channels, and region
By Drug Class: The biologics segment is dominating the disease-modifying therapies market with a 60.3% share in 2024

The market for disease-modifying treatments (DMT) is dominated by the biologics category for a number of important reasons. Compared to tiny compounds, biologics—such as fusion proteins and monoclonal antibodies—offer greater specificity and efficacy, precisely targeting disease processes. They work especially well for autoimmune conditions like Crohn's disease, RA, MS, and lupus. Patient outcomes are improved by the increasing use of monoclonal antibodies, their enhanced safety profiles, and their longer-lasting effects.
Additionally, innovations in biotechnology and an increase in regulatory approvals have expanded biologics availability globally. For instance, in August 2023, the FDA approved Tyruko (natalizumab‑sztn), developed by Polpharma Biologics and marketed by Sandoz, as the first and only biosimilar for relapsing forms of multiple sclerosis (MS), serving as a highly effective anti‑α4 integrin monoclonal antibody disease‑modifying treatment.
By Disease Indication: The multiple sclerosis segment is dominating the disease-modifying therapies market with a 40.3% share in 2024
The global market for disease-modifying therapies (DMT) is dominated by the Multiple Sclerosis (MS) segment for a number of important reasons. The need for efficient treatments is fueled by the high and rising prevalence of MS worldwide. Increased disease awareness and early diagnosis result in more patients starting DMTs. Continuous innovation helps the industry by providing improved efficacy and convenience through products including oral small molecules, monoclonal antibodies, and next-generation immunomodulators. Therapy that improves quality of life, delays advancement, and decreases relapses is preferred by patients, which increases adoption.
Furthermore, the continuous research and development and positive ongoing clinical trial results are driving market expansion. For instance, in May 2025, Roche reported that fenebrutinib, an investigational BTK inhibitor, maintained near-complete suppression of disease activity and prevented disability progression for up to two years in patients with relapsing multiple sclerosis, based on Phase II FENopta study results.
Disease-Modifying Therapies Market, Geographical Analysis

North America is dominating the global disease-modifying therapies market with 48.5% in 2024
North America dominates the Disease Modifying Therapies (DMT) market because of its high prevalence of multiple sclerosis, sophisticated healthcare system, and top pharmaceutical companies. The region's leadership in the worldwide DMT market has been strengthened by strong R&D investment, favorable reimbursement policies, early adoption of innovative DMTs, and growing availability of biosimilars.
In the U.S., the Disease Modifying Therapies (DMT) market expanded due to increased FDA approvals, priority reviews for novel therapies, and growing biosimilar availability, enhancing treatment outcomes and patient convenience worldwide. For instance, in September 2024, Roche announced that the U.S. FDA approved OCREVUS ZUNOVO (ocrelizumab & hyaluronidase-ocsq) for relapsing and primary progressive multiple sclerosis. It became the first and only twice‑a‑year, approximately 10‑minute subcutaneous injection, offering patients a more convenient treatment option.
Europe is the second region after North America, which is expected to dominate the global disease-modifying therapies market with 34.5% in 2024
In Europe, the market for disease-modifying therapies (DMTs) grew as a result of increased access to targeted treatments, early screening initiatives, and increased disease prevalence. Significant R&D expenditures, quick adoption of biologics and biosimilars, and strong European Commission approvals all contributed to market expansion, improving patient access and developing cutting-edge treatments throughout the region.
Moreover, recent EU approvals and new product launches for biologics significantly boosted market expansion and improved treatment availability across Europe. For instance, in May 2024, Biogen Inc. received European Commission approval for QALSODY (tofersen) to treat adults with SOD1‑ALS, maintaining orphan designation. It became the first therapy in the EU targeting the genetic cause of amyotrophic lateral sclerosis, offering a breakthrough for this rare condition.
The Asia-Pacific region is the fastest-growing region in the global disease-modifying therapies market, with a CAGR of 7.7% in 2024
The Asia-Pacific market for disease-modifying therapies (DMTs), which includes nations like China, Japan, South Korea, and India, expanded rapidly as a result of factors like increased disease prevalence, improved patient awareness, easier access to healthcare, a rise in the production of biologics and biosimilars, and robust government support for early diagnostic initiatives and targeted therapies.
Japan’s disease-modifying therapies market grew steadily, driven by rising disease prevalence, PMDA and Ministry of Health, Labour and Welfare approvals, new product launches, advanced biologics adoption, and strong collaborations with global biopharma companies improving patient access. Owing to factors like Ministry of Health, Labour and Welfare approvals, for instance, in December 2023, UCB announced that Japan’s Ministry of Health, Labour and Welfare approved BIMZELX (bimekizumab) for adults with psoriatic arthritis, non-radiographic axial spondyloarthritis, and ankylosing spondylitis unresponsive to existing treatments.
Disease-Modifying Therapies Market Competitive Landscape
Top companies in the disease-modifying therapies market include Genentech USA, Inc.; Novartis AG; Teva Neuroscience, Inc.; GSK; AstraZeneca; Johnson & Johnson; AbbVie Inc.; Amgen Inc.; UCB, Inc.; and Takeda Pharmaceuticals U.S.A., Inc., among others.
Genentech USA, Inc.: Genentech USA, Inc., a leading biotechnology company, focuses on developing innovative Disease Modifying Therapies (DMTs) for conditions like multiple sclerosis and neurodegenerative disorders. Leveraging advanced biologics, extensive R&D, and global clinical programs, the company aims to slow disease progression, improve patient outcomes, and expand access to cutting-edge therapies across the United States and worldwide.
Key Developments:
- In September 2025, Sanofi’s Tzield (teplizumab) was approved in China as the first disease‑modifying therapy for stage 2 type 1 diabetes. The NMPA granted priority approval, recognizing its innovative profile and benefit in delaying disease progression in patients aged eight and older.
- In February 2024, Roche Pharma India launched Ocrevus (ocrelizumab) for the treatment of multiple sclerosis, expanding its neurology portfolio and addressing the unmet needs of patients, particularly adults aged 20–40, who are most affected by this debilitating disease in India.
Market Scope
| Metrics | Details | |
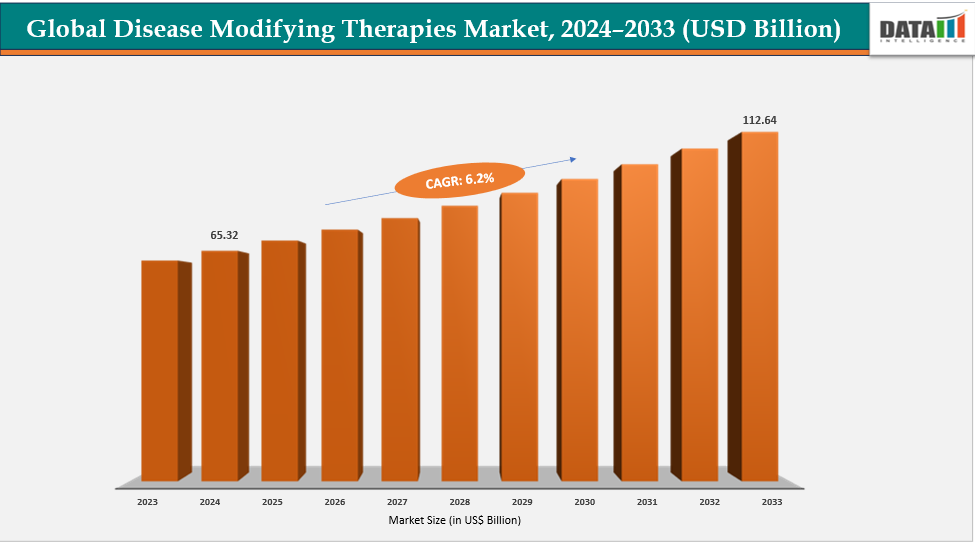
| CAGR | 6.2% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | By Drug Class | Biologics, Small Molecule Drugs |
| By Disease Indication | Multiple Sclerosis, Rheumatoid Arthritis, Lupus, Crohn’s Disease, and Other | |
| By Route of Administration | Parenteral, Oral | |
| By Distribution Channel | Hospital Pharmacies, Retail Pharmacies | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global disease-modifying therapies market report delivers a detailed analysis with 62 key tables, more than 53 visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more pharmaceutical-related reports, please click here