Desmoid Tumors Market Size& Industry Outlook
The global desmoid tumors market size reached US$1.88Billion in 2024 from US$1.79Billionin 2023 and is expected to reach US$ 3.05Billion by 2033, growing at a CAGR of 5.6%during the forecast period 2025-2033.
The market is an emerging niche within the rare oncology and soft-tissue sarcoma landscape, driven by rising awareness, improved molecular diagnostics, and the recent FDA approval of nirogacestat (OGSIVEO), the first targeted therapy specifically indicated for this disease. Despite its rarity, high per-patient treatment costs and orphan-drug pricing create strong commercial potential. However, treatment heterogeneity, slow disease progression, and limited global reimbursement still constrain broader market penetration.
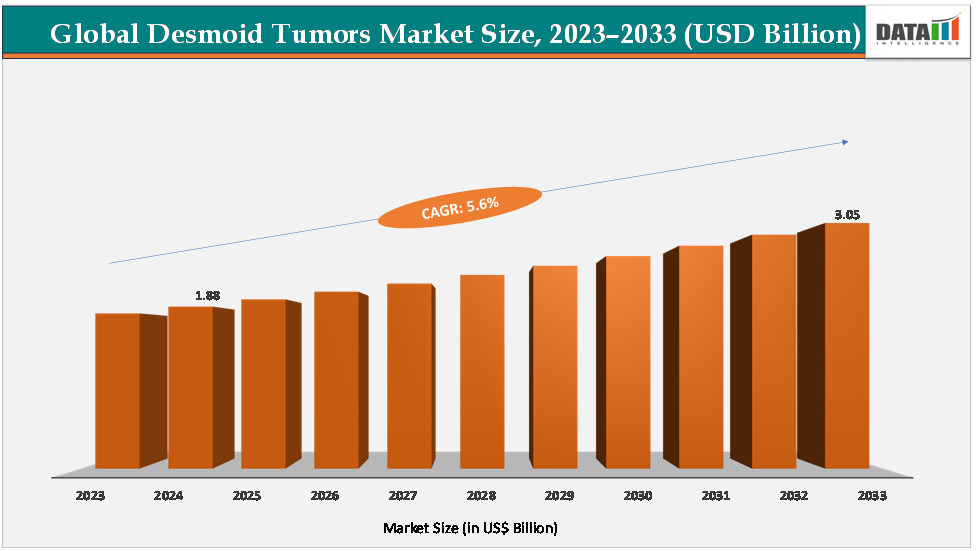
Key Market Highlights
- North America dominates the desmoid tumors market with the largest revenue share of 44.17% in 2024.
- The Asia Pacific is the fastest-growing region and is expected to grow at the fastest CAGR of5.7% over the forecast period.
- Based on treatment type, the targeted therapy segment led the market with the largest revenue share of 51.12% in 2024.
- The major market players in the desmoid tumors market are Spring Works Therapeutics, Inc., Immunome Inc., Apollomics, Inc., Iterion Therapeutics, Inc., and Parabilis Medicines, among others
Market Dynamics
Drivers: Expanding clinical pipeline is significantly driving the desmoid tumors market growth
The expanding clinical pipeline is a key growth catalyst for the desmoid tumors market, as it reflects both rising industry confidence and advancing scientific understanding of this once-overlooked rare tumors. The approval of nirogacestat (Ogsiveo) by Spring Works Therapeutics in 2023 validated the Notch/γ-secretase inhibition pathway, establishing proof of concept and encouraging broader R&D investments. Following this milestone, several companies have accelerated development programs targeting complementary mechanisms.
For instance, in October 2025, Parabilis Medicines announced at the ESMO Congress 2025 the first-ever clinical data showing that its investigational FOG-001 therapy has successfully drugged β-catenin:TCF – a key cancer-driving node in the Wnt/β-catenin pathway, until now considered “undruggable.” In the ongoing Phase 1/2 trial of FOG-001 in patients with a range of Wnt/β-catenin-driven tumors, as of mid-August 2025, 12 patients with desmoid tumors had been dosed across three dose levels.
Similarly, in February 2024, Immunome, Inc. announced that it entered into a definitive asset purchase agreement with Ayala Pharmaceuticals, Inc., a clinical-stage oncology company, to acquire AL102 and related drug candidate AL101 from Ayala. Based on the terms of the agreement, Immunome will pay Ayala $20 million in cash and $30 million in Immunome common stock (valued at 30-day VWAP as of February 1, 2024) at the closing and will pay up to an additional $37.5 million in development and commercial milestone payments. Completion of the transaction is subject to customary conditions including Ayala obtaining the requisite stockholder approval.
These diverse clinical programs increase the likelihood of multiple approved agents, broadening therapeutic choice and access. Moreover, these ongoing academic–industry collaborations and patient registries are enhancing data collection, facilitating trial recruitment, and supporting adaptive study designs in this rare population. Collectively, this expanding and mechanistically varied pipeline and collaborations not only boosts investor confidence but also promises improved efficacy, tolerability, and quality of life for patients ultimately driving sustained market growth through innovation and indication expansion.
Restraints: Availability of limited approved drugs are hampering the growth of the market
The limited availability of approved drugs with nirogacestat (Ogsiveo) currently being the only FDA-approved therapy for desmoid tumors acts as a significant restraint on market growth. While its 2023 approval marked a major breakthrough, dependence on a single branded therapy restricts treatment options, limits competition, and sustains high pricing, creating access and reimbursement challenges in many countries.
Physicians often resort to off-label use of older agents such as sorafenib, imatinib, or pazopanib, which have variable efficacy and safety profiles, leading to inconsistent clinical outcomes. Moreover, the absence of alternative approved therapies reduces prescriber confidence and patient choice, especially for those who experience adverse effects or relapse after nirogacestat treatment. The lack of multiple entrants also discourages wider geographic adoption, as some regions delay reimbursement for single-source orphan drugs. This narrow therapeutic landscape constrains overall treatment uptake and underscores the urgent need for diversified approvals and competitive pricing to unlock the full commercial potential of the desmoid tumors market.
For more details on this report – Request for Sample
Desmoid Tumors Market, Segmentation Analysis
The global desmoid tumors market is segmented based on tumor type, treatment type, end-user, and region.
Treatment Type:
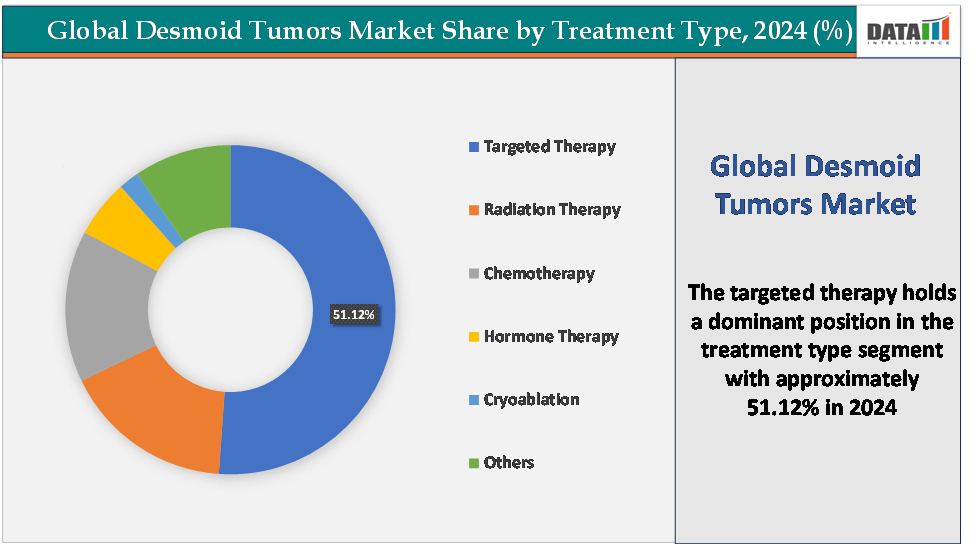
The targeted therapy segment is dominating and fastest-growing in the desmoid tumors market with a 51.12% share in 2024
The targeted therapy segment is currently the dominant and fastest-growing area in the desmoid tumors market, driven by advances in molecular biology and the emergence of drugs that precisely address the disease’s underlying genetic and signaling abnormalities. The approval of nirogacestat (Ogsiveo) by Spring Works Therapeutics in 2023 revolutionized treatment by introducing the first γ-secretase inhibitor that blocks aberrant Notch signaling, a key pathway in desmoid tumor proliferation. This landmark approval validated the therapeutic potential of targeted approaches and shifted clinical practice away from non-specific chemotherapies and surgery toward precision medicine.
Following this success, several targeted agents are advancing through the pipeline, including Immunome’s AL102 (another γ-secretase inhibitor in Phase 3 trials) and Iterion Therapeutics’ tegavivint, which disrupts the β-catenin/TBL1 interaction central to Wnt signaling. Additionally, Parabilis Medicines’ FOG-001 and Cellestia Biotech’s CB-103 represent next-generation inhibitors acting on the Wnt/β-catenin and Notch pathways, respectively. These pipeline therapies are expected to expand indications, improve tolerability, and offer alternative mechanisms for patients resistant to current treatments.
The success of nirogacestat has also drawn attention from major pharma players, who are exploring multi-kinase inhibitors such as sorafenib and pazopanib for off-label or label-expansion opportunities. Collectively, this rapid innovation and validation of molecularly targeted strategies position the targeted therapy segment as the central growth engine of the desmoid tumor market, expected to capture the largest market share over the next decade.
Geographical Analysis

North America is dominating the global desmoid tumors market with a 44.17% in 2024
North America dominates the global desmoid tumors market due to its presence of major market players, strong regulatory framework, and early adoption of innovative therapies. High disease awareness, active patient advocacy groups, and robust R&D investment from biotech companies further drive market growth and clinical trial activity across the region.
US Desmoid Tumors Market Trends
The United States holds a dominant position in the global desmoid tumors market, driven by its strong regulatory support, rapid adoption of innovative therapies, and concentrated R&D activity. The FDA’s 2023 approval of nirogacestat (Ogsiveo) by Spring Works Therapeutics marked a historic milestone, making the U.S. the first and only country with an approved targeted therapy for desmoid tumors. This early regulatory success has attracted significant investment and clinical trial activity, with multiple biotech companies including Immunome, Iterion Therapeutics, and Parabilis Medicines conducting pivotal studies across leading U.S. cancer centers.
Additionally, the country benefits from a well-established rare disease ecosystem, with supportive reimbursement frameworks, active patient advocacy through organizations like the Desmoid Tumor Research Foundation (DTRF), and strong collaboration between academia and industry. High disease awareness, advanced diagnostic capabilities, and premium orphan-drug pricing further reinforce the U.S. market’s leadership, making it the largest revenue contributor and innovation hub in the global desmoid tumor landscape.
The Asia Pacific region is the fastest-growing region in the global desmoid tumors market, with a CAGR of 5.7% in 2024
The Asia-Pacific region is emerging as the fastest-growing market in the global desmoid tumors landscape, driven by rapid improvements in healthcare infrastructure, increased disease awareness, and expanding clinical research activity across major economies such as China, Japan, India, and South Korea. Although the region currently lags behind North America and Europe in terms of approved therapies, it is witnessing exponential growth in diagnostic capabilities and access to advanced oncology care, which are crucial for early identification and management of rare diseases like desmoid tumors.
Rising government support for orphan disease treatment and inclusion of rare cancers in national healthcare policies have accelerated investment and collaboration between global biopharma companies and local research institutes. For instance, several multinational firms, including Spring Works Therapeutics and Immunome, are expanding clinical trial sites in Asia-Pacific to support ongoing studies of γ-secretase inhibitors such as nirogacestat and AL102, reflecting the region’s growing role in global R&D.
Europe Desmoid Tumors Market Trends
The growth of the desmoid tumors market in Europe is being propelled by a distinct set of regulatory, clinical-practice and commercial developments that together are creating momentum across the region. The Europe regulatory breakthrough validates the market and opens commercial pathways across EU member states. The approval of OGSIVEO in Europe marks the first-ever approved therapy for desmoid tumors in Europe, filling a major unmet need and enabling systemic therapy uptake rather than relying solely on surgery or watch-and-wait.
For instance, in August 2025, Merck, a leading science and technology company, announced that the European Commission (EC) granted marketing authorization for OGSIVEO (nirogacestat), an oral gamma secretase inhibitor, as monotherapy for the treatment of adults with progressing desmoid tumors who require systemic treatment. OGSIVEO is the first and only therapy approved in the European Union (EU) to treat desmoid tumors. The approval was issued to Spring Works Therapeutics Inc., a healthcare company of Merck.
Competitive Landscape
Top companies in the desmoid tumors market include Spring Works Therapeutics, Inc., Immunome Inc., Apollomics, Inc., Iterion Therapeutics, Inc., and Parabilis Medicines, among others.
Desmoid Tumors Market Scope
| Metrics | Details | |
| CAGR | 5.6% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Tumor Type | Abdominal Wall Desmoid Tumors, Intra-Abdominal Desmoid Tumors, and Extra-Abdominal Desmoid Tumors |
| Treatment Type | Targeted Therapy, Radiation Therapy, Chemotherapy, Hormone Therapy, Cryoablation, and Others | |
| End-User | Hospitals, Specialty Clinics, Ambulatory Surgical Centers, and Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America and the Middle East & Africa | |
The global desmoid tumors market report delivers a detailed analysis with 47 key tables, more than 51visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.