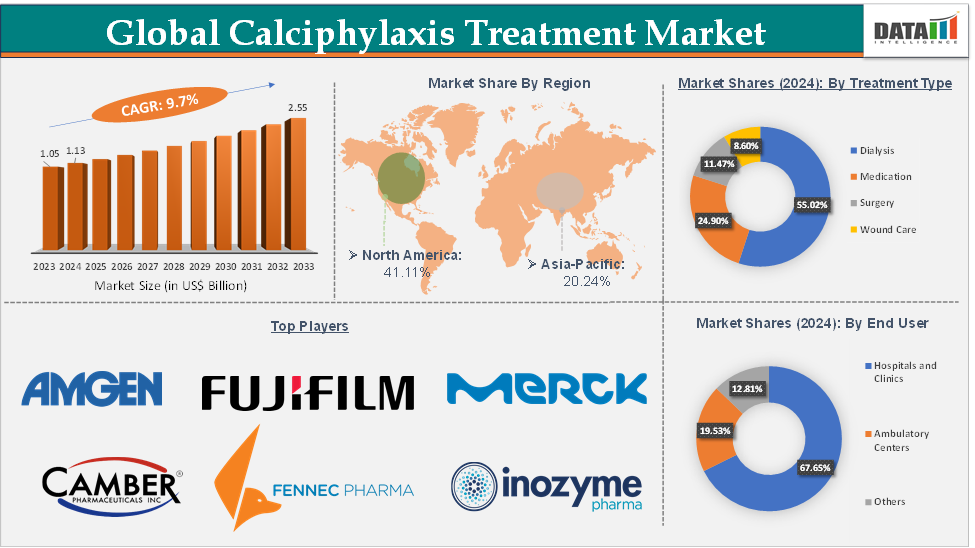
Calciphylaxis Treatment Market Size
The global calciphylaxis treatment market size reached US$ 1.13 Billion in 2024 from US$ 1.05 Billion in 2023 and is expected to reach US$ 2.55 Billion by 2033, growing at a CAGR of 9.7% during the forecast period 2025-2033.
Calciphylaxis Treatment Market Overview
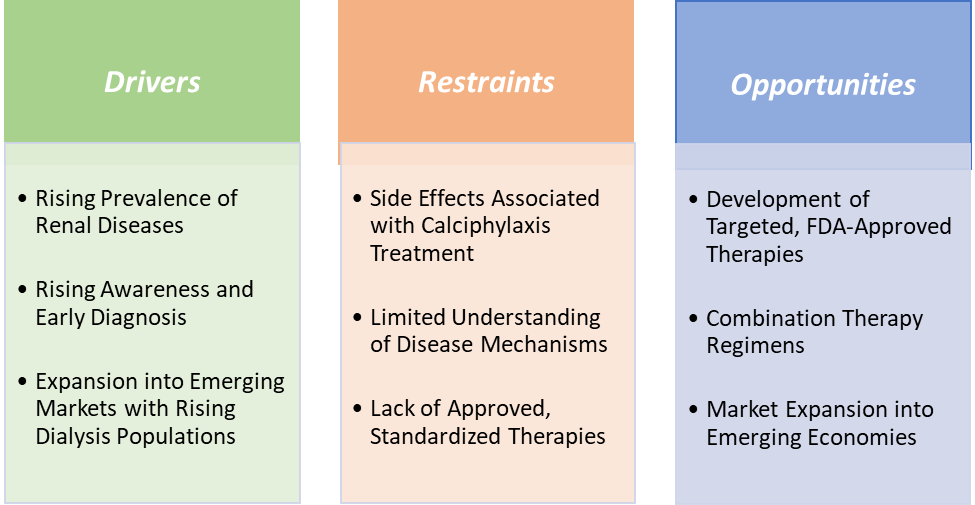
Owing to factors such as the rising prevalence of renal diseases, increasing awareness about the condition and early diagnosis, rising clinical trials to develop more therapeutics for the treatment of calciphylaxis, and rising advancements in the field of calciphylaxis treatment are expected to drive the market over the forecast period. Additionally, adverse effects and complications associated with the calciphylaxis treatment, high cost associated with the treatment, availability of limited treatment options and limited understanding of disease mechanisms are the factors expected to hamper the market growth over the forecast period.
Calciphylaxis Treatment Market Executive Summary
Calciphylaxis Treatment Market Dynamics
Drivers:
As the incidence of renal diseases such as chronic kidney disease and end-stage renal disease continues to rise globally, there is a larger population of individuals at risk for developing calciphylaxis. Renal diseases are known risk factors for calciphylaxis, particularly among patients undergoing long-term dialysis treatment.
For instance, according to the International Society of Nephrology, a fact that underlines that there are more than 850 million people who suffer from some form of kidney disease, which is increasing every year. The prevalence of CKD worldwide is 10.4% among men and 11.8% among women. Acute Kidney Injury (AKI), experienced by 13.3 million people each year, may resolve or lead to CKD or kidney failure in the future. With more individuals affected by renal diseases, there is a corresponding increase in the incidence of calciphylaxis.
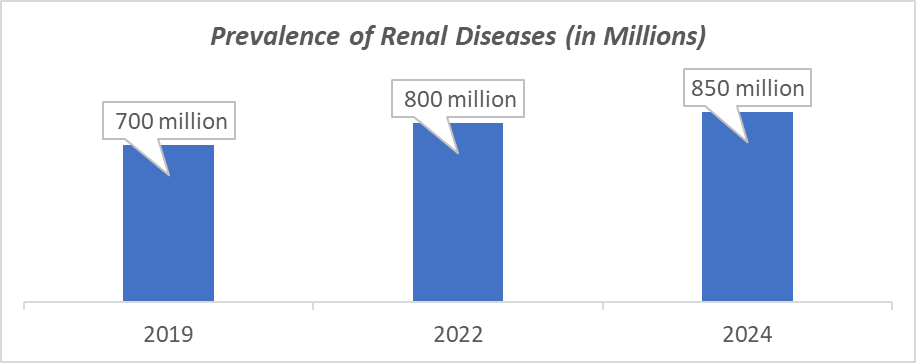
Source: International Society of Nephrology
According to the United States Renal Data System, there were 124,411 new ESRD diagnoses, reflecting an increasing burden of kidney failure. The prevalence of the disease has been rising at a stable rate of about 20,000 cases per year. Kidney disease is the ninth leading cause of death in the United States. The growing burden of calciphylaxis underscores the need for effective treatments to manage this condition. Healthcare providers are increasingly recognizing calciphylaxis as a significant clinical challenge and are seeking better therapeutic options to improve patient outcomes.
Restraints:
Side effects associated with calciphylaxis treatment are hampering the growth of the market
Many of the treatments for calciphylaxis, such as sodium thiosulfate and hyperbaric oxygen therapy, may have side effects and risks associated with their use. For instance, sodium thiosulfate can cause nausea, vomiting, and metabolic acidosis, while hyperbaric oxygen therapy may lead to ear barotrauma or oxygen toxicity. These side effects can impact patient tolerance and adherence to treatment, potentially leading to treatment discontinuation or suboptimal outcomes.
Some calciphylaxis treatments, particularly pharmacological interventions, carry a risk of serious adverse events. For instance, sodium thiosulfate administration may be associated with hypotension, allergic reactions, or electrolyte disturbances. The occurrence of adverse events can necessitate close monitoring, dose adjustments, or additional interventions, increasing the complexity and cost of treatment.
Side effects of calciphylaxis treatment can negatively affect patients' quality of life, contributing to physical discomfort, psychological distress, and impaired functional status. Pain, nausea, fatigue, and other treatment-related symptoms may diminish patient well-being and limit daily activities, leading to decreased treatment compliance and satisfaction.
In addition, serious adverse events associated with calciphylaxis treatments can raise safety concerns among healthcare providers, patients and regulatory authorities. Instances of treatment-related complications may prompt regulatory scrutiny, leading to increased oversight, labeling changes, or even restrictions on the use of certain therapies. This could potentially impact market access and adoption of calciphylaxis treatments.
Opportunities:
Development of targeted, FDA-approved therapies creates a market opportunity for the calciphylaxis treatment market
Calciphylaxis currently lacks any FDA-approved, disease-specific treatments, resulting in reliance on off-label therapies like sodium thiosulfate, which have variable efficacy and safety profiles. This significant unmet medical need creates a promising opportunity for pharmaceutical companies to develop targeted, approved therapies that can improve patient outcomes and reduce mortality.
SNF472, a novel intravenous inhibitor of vascular calcification currently in late-stage clinical trials for calciphylaxis, is a key instance. If approved, it would be the first targeted therapy for this condition, potentially capturing the entire calciphylaxis treatment market segment. By addressing the lack of approved options, targeted therapies provide clinically meaningful benefits, attract investment via orphan drug pathways, and open avenues for market growth through premium pricing and improved patient outcomes, making their development a crucial market opportunity.
Calciphylaxis Treatment Market Trends
For more details on this report – Request for Sample
Calciphylaxis Treatment Market, Segment Analysis
The global calciphylaxis treatment market is segmented based on treatment type, end-user, and region.
The dialysis segment from the treatment type reached US$ 620.50 Million in 2024 in the calciphylaxis treatment market
Dialysis is a vital component of the treatment regimen for patients with end-stage renal disease (ESRD) or advanced chronic kidney disease (CKD). Calciphylaxis is strongly associated with end-stage renal disease (ESRD) and chronic kidney disease (CKD), particularly in patients on long-term hemodialysis. As such, dialysis plays a crucial role in managing the underlying renal dysfunction that contributes to the development and progression of calciphylaxis. The increasing prevalence of ESRD and CKD, driven by factors such as aging populations and rising rates of diabetes and hypertension, contributes to the growing demand for dialysis services in the context of calciphylaxis treatment.
For instance, according to the National Kidney Foundation, around 10% of the population worldwide is affected by chronic kidney disease (CKD), and millions die each year because they do not have access to affordable treatment. Over 2 million people worldwide currently receive treatment with dialysis or a kidney transplant to stay alive, yet this number may only represent 10% of people who actually need treatment to live.
Calciphylaxis Treatment Market, Geographical Analysis
North America is expected to dominate the global calciphylaxis treatment market with a US$ 463.64 Million in 2024
The North American region is expected to grow during the forecast period owing to the presence of a patient population, well-developed technology, high healthcare expenditure, the focus of research institutions on updating new versions of treatment options and the presence of the leading players. Furthermore, modern hospital infrastructures and the availability of experienced healthcare personnel are fueling market expansion.
Due to the high frequency of end-stage renal disease (ESKD), one of the leading causes of calciphylaxis in the United States. According to the Regents of the University of California, the rate of ESKD in the United States is increasing by 5% per year. Calciphylaxis primarily affects dialysis patients; this might be another crucial factor favorably influencing market growth in the next years.
Furthermore, according to the National Institutes of Health, more than 808,000 people in the United States are living with ESKD, also known as end-stage renal disease (ESRD), with 68% on dialysis. Men are 1.6 times more likely to develop ESKD than women. This could be a significant element in the growth of the global market for calciphylaxis treatment.
Furthermore, continuing research and clinical trials in the area focus on creating targeted therapeutics and new treatment options for calciphylaxis, reinforcing North America's position as the worldwide market leader. For instance, in January 2025, Inozyme Pharma, Inc. announced positive interim data from its ENERGY 1 trial and Expanded Access Program (EAP) evaluating INZ-701 in infants and young children. INZ-701 is an ENPP1 Fc fusion protein enzyme replacement therapy (ERT) designed to increase PPi and adenosine, enabling the potential treatment of multiple diseases caused by deficiencies in these molecules. It is currently in clinical development for the treatment of calciphylaxis.
Asia-Pacific is growing at the fastest pace in the calciphylaxis treatment market, with a US$ 228.24 Million in 2024
The Asia Pacific is one of the fastest growing calciphylaxis market on the basis of a larger number of developing nations present in this region, countries such as India, Japan and China. These countries are encouraging the growth of the healthcare field. The increasing patient population of cancer in developing countries will directly raise the demand for various diagnoses & treatment methods, which will help in boosting the market growth.
There was substantial variation in overall and advanced chronic kidney disease prevalence, up to an estimated 434.3 million, where adults have chronic kidney disease in Asia, including up to 65.6 million (95% CI 42.2 to 94.9) who have advanced chronic kidney disease. The greatest number of adults living with chronic kidney disease were in China (up to 159.8 million, 95% CI 146.6 to 174.1) and India (up to 140.2 million, 95% CI 110.7 to 169.7), collectively having 69.1% of the total number of adults with chronic kidney disease in the region. Hence, the large number of people with chronic kidney disease, and the substantial number with advanced chronic kidney disease, increases the demand for calciphylaxis, which helps the overall country and region to grow during the forecast period.
In India, the prevalence of chronic kidney disease has increased to epidemic proportions and population-based studies have reported a 4%–20% prevalence of chronic kidney disease in India. chronic kidney disease in India is often diagnosed late and due to the lack of awareness often plays an important role, and the absence of symptoms (higher incidence of interstitial nephritis and chronic kidney disease of unknown origin) contributes to the delay. The burden of kidney failure is increasing, with almost 2,10,000 new cases being diagnosed each year.
Calciphylaxis Treatment Market Competitive Landscape
Top companies in the calciphylaxis treatment market include Amgen Inc., Merck KGaA, Sun Pharmaceutical Industries Ltd, Fennec Pharmaceuticals Inc., Camber Pharmaceuticals, Inc., Hope Pharmaceuticals, FUJIFILM Corporation, Dr. Reddy’s Laboratories Limited, Aurobindo Pharma Limited, and Inozyme Pharma, Inc., among others.
Calciphylaxis Treatment Market Scope
Metrics | Details | |
CAGR | 9.7% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Treatment Type | Dialysis, Medication, Surgery, and Wound Care |
End-User | Hospitals and Clinics, Ambulatory Centers, and Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
DMI Insights on Calciphylaxis Treatment Market
According to the DMI analysis, the global calciphylaxis treatment market size reached US$ 1.13 Billion in 2024 and is expected to reach US$ 2.55 Billion by 2033, growing at a CAGR of 9.7% during the forecast period 2025-2033.
The calciphylaxis treatment market represents a highly specialized and emerging segment driven by a critical unmet need in patients with end-stage renal disease (ESRD) and chronic kidney disease (CKD). Despite being a rare and often fatal condition with mortality rates reaching up to 80% within six months, the market currently lacks any FDA-approved targeted therapies, relying largely on off-label treatments such as sodium thiosulfate and wound care.
The growing global prevalence of CKD and dialysis patients fuels the potential patient pool, while advances in understanding the disease’s complex pathophysiology open doors for the development of novel, targeted therapies. The introduction of drugs like SNF472, which inhibit vascular calcification, could revolutionize treatment and significantly improve patient survival and quality of life.
However, the market faces several challenges, including diagnostic delays, physician awareness gaps, and high treatment costs coupled with reimbursement hurdles. The small patient population limits commercial scale, demanding strategic pricing and strong clinical evidence to justify market adoption. Moreover, the heterogeneity in patient response necessitates personalized and multidisciplinary approaches.
The global calciphylaxis treatment market report delivers a detailed analysis with 54 key tables, more than 43 visually impactful figures, and 135 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more pharmaceutical-related reports, please click here