ANGPTL3-Lowering Therapy Market - Industry Trends & Overview
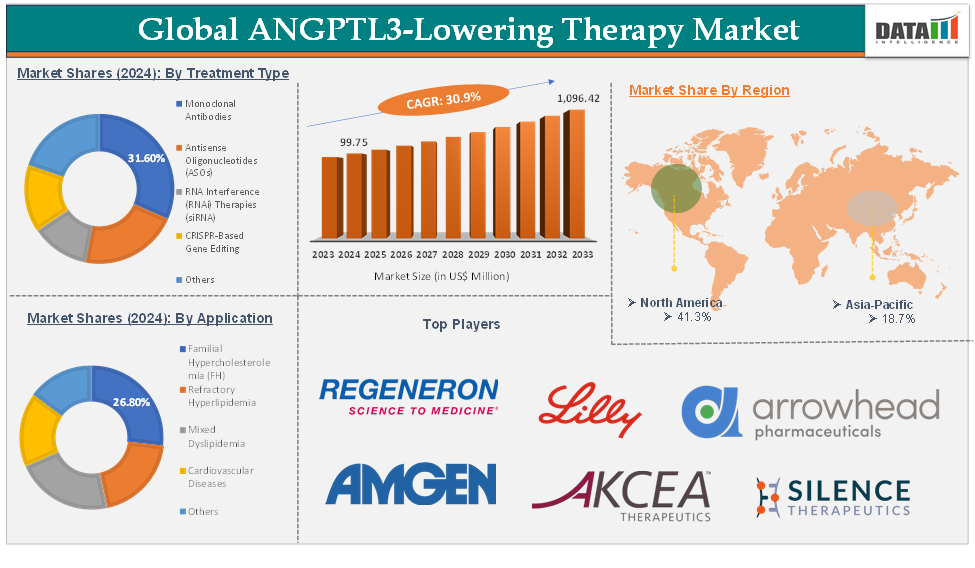
ANGPTL3-Lowering Therapy Market reached US$ 99.75 Million in 2024 and is expected to reach US$ 1,096.42Million by 2033, growing at a CAGR of 30.9% during the forecast period 2025-2033.
ANGPTL3-lowering therapy refers to a class of treatments designed to reduce the activity or levels of angiopoietin-like protein 3 (ANGPTL3), a protein produced exclusively in the liver that plays a central role in regulating lipid and lipoprotein metabolism.
The global ANGPTL3-lowering therapy market is poised for rapid expansion, driven by the increasing prevalence of hyperlipidemia and cardiovascular diseases (CVD) worldwide. Traditional therapies like statins and PCSK9 inhibitors often fall short in patients with severe or genetic lipid disorders, such as homozygous familial hypercholesterolemia (HoFH), creating a significant unmet medical need.
ANGPTL3 inhibitors have emerged as a promising new class of therapies that target this protein to reduce LDL-C and triglycerides more effectively, offering hope for patients with difficult-to-manage lipid profiles. Key pharmaceutical companies, including Arrowhead Pharmaceuticals, Eli Lilly, Verve Therapeutics, and Regeneron, are actively developing and launching novel ANGPTL3-targeting therapies that are expected to further accelerate market growth.
The approval of drugs like Evkeeza (evinacumab) for both adults and pediatric patients with HoFH exemplifies the expanding treatment options and regulatory support. Additionally, ongoing research highlights the critical role of ANGPTL3 in lipid metabolism and cardiovascular risk, reinforcing the therapeutic potential of its inhibition.
Executive Summary
For more details on this report – Request for Sample
ANGPTL3-Lowering Therapy Market Dynamics: Drivers
Rising prevalence of hyperlipidemia and cardiovascular diseases (CVD)
The rising prevalence of hyperlipidemia, a major contributor to cardiovascular diseases (CVD), is fueling the demand for effective lipid-lowering treatments, particularly those that target the ANGPTL3-lowering therapy. Inhibitors of ANGPTL3 have emerged as a promising therapeutic option for controlling lipid levels, especially in patients who do not adequately respond to conventional therapies. These inhibitors are particularly beneficial for individuals with difficult-to-manage hyperlipidemia, offering an alternative approach to lipid management that addresses unmet clinical needs in this patient population.
Cardiovascular disease (CVD) & hyperlipidemia stand as the leading causes of mortality worldwide, heavily influenced by elevated plasma levels of low-density lipoprotein cholesterol (LDL-C) and the resultant formation of atherosclerotic plaques.
Physicians have access to various LDL-C-lowering medications, including statins, ezetimibe, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, and inclisiran. Recently, angiopoietin-like protein 3 (ANGPTL3) inhibitors have emerged as a significant addition to lipid-lowering therapies, particularly for patients with challenging cases of hypercholesterolemia, such as those with homozygous familial hypercholesterolemia (HoFH). Thus, all these factors drive the ANGPTL3-lowering therapy market growth.
According to the research study data in the New England Journal of Medicine in May 2024, Angiopoietin-like 3 (ANGPTL3) acts to inhibit both lipoprotein and endothelial lipases, as well as the liver’s ability to clear triglyceride-rich lipoprotein remnants. Individuals with loss-of-function mutations in ANGPTL3 have lower levels of triglycerides, LDL cholesterol, HDL cholesterol, and non-HDL cholesterol, and are at reduced risk for atherosclerotic cardiovascular disease compared to those without such mutations. Zodasiran is an RNA interference (RNAi) therapy designed to suppress ANGPTL3 production in the liver.
ANGPTL3-Lowering Therapy Market Dynamics: Restraints
Availability of limited treatment options
The limited availability of treatment options presents a significant challenge in the global ANGPTL3-lowering therapy market, particularly for patients suffering from conditions like homozygous familial hypercholesterolemia (HoFH).
As per Ultragenyx Pharmaceutical Inc. news in January 2024, Homozygous familial hypercholesterolemia (HoFH) is a severe form of inherited hypercholesterolemia that affects approximately 1 in 300,000 individuals worldwide and around 1,600 people in the European Union. This condition arises when a person inherits two copies of genes that cause familial hypercholesterolemia (FH), one from each parent, resulting in dangerously high levels of low-density lipoprotein cholesterol (LDL-C), often exceeding 400 mg/dL.
Currently, evinacumab is primarily utilized as an adjunct therapy alongside existing lipid-lowering treatments, including statins and PCSK9 inhibitors. Many patients with HoFH are already on multiple lipid-lowering medications, but the effectiveness of these combinations can vary significantly due to individual genetic differences. For instance, patients lacking functional LDL receptors may not respond to PCSK9 inhibitors, further constraining the therapeutic landscape. Thus, the above factors could be limiting the global ANGPTL3-lowering therapy market's potential growth.
ANGPTL3-Lowering Therapy Market Segment Analysis
The global ANGPTL3-lowering therapy market is segmented based on treatment type, application, and region.
Treatment Type:
The monoclonal antibodies treatment type segment is expected to hold 31.6% of the global ANGPTL3-lowering therapy market in 2024
Evkeeza (evinacumab-dgnb) is a medication classified as a monoclonal antibody, which means it is a laboratory-made protein designed to target a specific molecule in the body. In this case, Evkeeza binds to and blocks the activity of angiopoietin-like protein 3 (ANGPTL3). ANGPTL3 is a naturally occurring protein that plays a key role in regulating lipid (fat) metabolism. It does this by inhibiting certain enzymes, such as lipoprotein lipase and endothelial lipase, that are responsible for breaking down fats in the bloodstream.
By inhibiting ANGPTL3, Evkeeza effectively removes this “brake” on fat breakdown. This allows the enzymes to work more efficiently, leading to a reduction in levels of triglycerides and cholesterol, including low-density lipoprotein (LDL) cholesterol, often referred to as “bad” cholesterol.
Evkeeza is particularly useful for patients with rare genetic conditions, such as homozygous familial hypercholesterolemia (HoFH), who have extremely high cholesterol levels that are difficult to control with standard therapies. By targeting ANGPTL3, Evkeeza offers a novel approach to lowering harmful blood fats and reducing cardiovascular risk in these high-need patients.
For instance, in January 2024, Ultragenyx Pharmaceutical Inc. announced that the National Institute for Health and Care Excellence (NICE) had released a final draft guidance recommending Evkeeza (evinacumab) for use within NHS England. Evkeeza is advised as an adjunct to diet and other therapies that lower low-density lipoprotein cholesterol (LDL-C) for treating adults and adolescents aged 12 years and older diagnosed with homozygous familial hypercholesterolemia (HoFH). These factors have solidified the segment's position in the global ANGPTL3-lowering therapy market.
ANGPTL3-Lowering Therapy Market Geographical Analysis
North America is expected to hold 41.3% of the global ANGPTL3-lowering therapy market in 2024
The North American market for ANGPTL3-lowering therapies is expanding rapidly, primarily due to the high rates of hyperlipidemia and cardiovascular diseases such as atherosclerosis in the region. Many patients continue to struggle with elevated cholesterol and triglyceride levels despite using standard treatments like statins, creating a strong need for more effective therapies.
This demand is further fuelled by growing awareness of cardiovascular risk factors and the importance of managing cholesterol to prevent heart disease. The market is also being driven by the presence of leading pharmaceutical companies, frequent new product launches, and regulatory approvals for innovative drugs, such as Evkeeza for rare genetic cholesterol disorders.
Additionally, advances in cutting-edge technologies, including RNA interference, monoclonal antibodies, and gene editing, are providing new treatment options for patients with difficult-to-treat or inherited lipid disorders. North America’s advanced healthcare infrastructure and significant investment in research and development further strengthen its leadership in the global ANGPTL3-lowering therapy market.
For instance, in March 2025, Verve Therapeutics announced that the U.S. Food and Drug Administration (FDA) had cleared its Investigational New Drug (IND) application for VERVE-102, allowing the company to begin clinical trials in the United States. VERVE-102 is an innovative, investigational gene-editing therapy designed for patients with heterozygous familial hypercholesterolemia (HeFH), a genetic disorder that causes very high cholesterol levels and/or premature coronary artery disease (CAD). Thus, the above factors are consolidating the region's position as a dominant force in the global ANGPTL3-lowering therapy market.
Asia-Pacific is expected to hold 18.7% of the global ANGPTL3-lowering therapy market in 2024
The rising incidence of hyperlipidemia is a significant factor driving the ANGPTL3-lowering therapy market. According to Heart Foundation data in January 2024, more than 2 in 5 (42%) of Australian adults are affected by high cholesterol, with the condition being most prevalent among individuals aged 55 to 64 years. Despite this significant statistic, only 7% of adults identify high cholesterol as a major risk factor for heart disease.
Additionally, increasing awareness about the importance of cholesterol management and preventive healthcare is encouraging more patients and healthcare providers to seek out innovative treatment options. The region is also seeing increased investment in healthcare infrastructure, research, and development, with several countries promoting clinical trials and the adoption of new therapies. Pharmaceutical companies are expanding their presence in Asia Pacific, introducing advanced drugs such as monoclonal antibodies and RNA interference therapies to address unmet medical needs.
ANGPTL3-Lowering Therapy Market Major Players
The major global players in the ANGPTL3-lowering therapy market include Regeneron Pharmaceuticals, Inc., Arrowhead Pharmaceuticals Inc., Amgen Inc., Ionis Pharmaceuticals, Inc. (Akcea Therapeutics), Silence Therapeutics, Eli Lilly and Company, Novartis AG, Verve Therapeutics, Inc., and CRISPR Therapeutics, among others.
Key Developments
In May 2024, Arrowhead Pharmaceuticals recently presented new Phase 2 clinical data for Zodasiran (formerly known as ARO-ANG3), an investigational RNA interference (RNAi) therapy being developed for patients with mixed hyperlipidemia, a condition characterized by elevated levels of multiple types of blood lipids, such as cholesterol and triglycerides. Zodasiran works by targeting and reducing the production of angiopoietin-like protein 3 (ANGPTL3), a protein made in the liver that plays a key role in regulating lipid and lipoprotein metabolism.
Market Scope
Metrics | Details | |
CAGR | 30.93% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Treatment Type | Monoclonal Antibodies, Antisense Oligonucleotides (ASOs) RNA Interference (RNAi) Therapies (siRNA), CRISPR-Based Gene Editing, Others |
Application | Familial Hypercholesterolemia (FH), Refractory Hyperlipidemia, Mixed Dyslipidemia, Cardiovascular Diseases, Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and Middle East & Africa | |