Disease Overview:
Alpha-1 antitrypsin deficiency (AATD) is a hereditary disorder that results in insufficient production or malfunction of the alpha-1 antitrypsin (AAT) protein. This protein normally shields tissues from damage caused by neutrophil elastase, an enzyme released during immune responses to inflammation or infection. In people with AAT deficiency, the low levels or dysfunction of AAT allow this enzyme to break down lung tissue, contributing to respiratory conditions such as emphysema and bronchiectasis. Moreover, misfolded AAT can accumulate in the liver, potentially leading to liver injury over time.
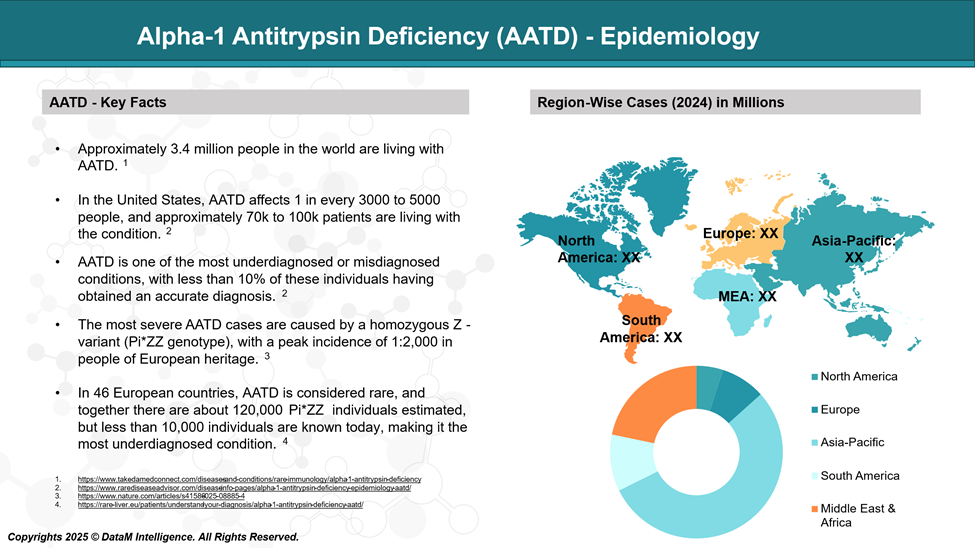
Epidemiology Analysis (Current & Forecast)
AATD is estimated to affect approximately 117 million people globally as carriers, with around 3.4 million individuals developing the condition.
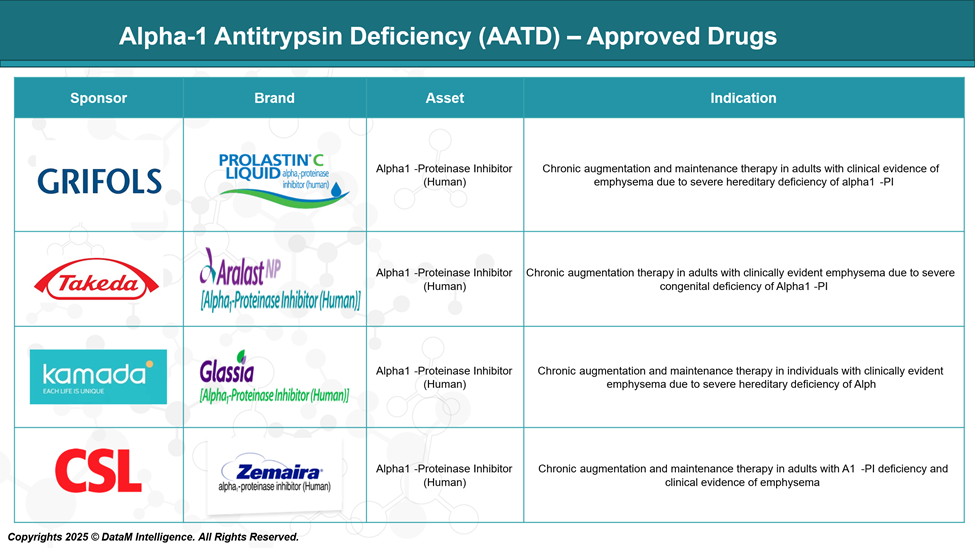
Approved Drugs - Sales & Forecast
Currently, several FDA-approved medications are available for the treatment of AATD, primarily focusing on augmentation therapy. This therapy involves intravenous administration of alpha-1 proteinase inhibitors derived from human plasma to increase AAT levels in the blood and lungs, aiming to slow the progression of emphysema associated with AATD.
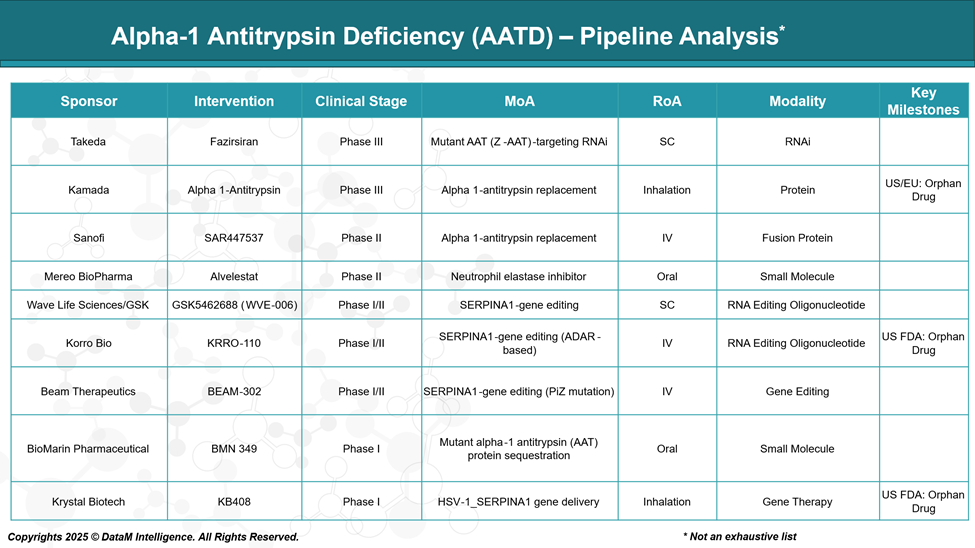
Pipeline Analysis and Expected Approval Timelines
The therapeutic landscape for AATD is rapidly evolving, with a diverse pipeline of investigational treatments targeting both pulmonary and hepatic manifestations of the disease. The emerging therapies encompass a range of innovative approaches, such as gene editing and gene therapy beyond traditional augmentation therapy.
Competitive Landscape and Market Positioning
The AATD market is evolving from traditional protein replacement therapies to innovative approaches like RNAi, gene editing, and gene therapy. This shift is reshaping competitive dynamics as emerging therapies aim for improved efficacy, convenience, and disease modification.
Company | Product (Status) | Modality | Mechanism / Target | Key Differentiator / Strategic Insight |
Grifols | Prolastin-C (Approved) | Plasma-derived protein | AAT replacement | Legacy therapy, standard of care, weekly IV dosing |
Takeda | Aralast NP (Approved) | Plasma-derived protein | AAT replacement | Competes with Prolastin; strong U.S. presence |
Kamada | Glassia (Approved) | Plasma-derived protein | AAT replacement | Ready-to-use liquid; streamlined preparation |
CSL Behring | Zemaira (Approved) | Plasma-derived protein | AAT replacement | High purity; tailored for long-term maintenance |
Takeda | Fazirsiran (Phase III) | RNA interference (RNAi) | Silences mutant Z-AAT mRNA | First disease-modifying therapy; dual liver and lung benefit |
Kamada | Inhaled AAT (Phase III) | Protein replacement | AAT augmentation | First inhaled AAT; targeted lung delivery |
Sanofi | SAR447537 (Phase II) | Fusion protein | Recombinant AAT | Monthly dosing potential, longer half-life |
Mereo BioPharma | Alvelestat (Phase II) | Small molecule | Neutrophil elastase inhibitor | Non-replacement; targets inflammation; oral therapy |
Wave Life Sciences / GSK | GSK5462688 (WVE-006) (Phase I/II) | RNA editing oligonucleotide | Edits SERPINA1 gene (mRNA correction) | First-in-human RNA editing: precise, reversible correction |
Korro Bio | KRRO-110 (Phase I/II) | RNA editing oligonucleotide | ADAR-mediated RNA editing of SERPINA1 | Precision editing avoids permanent DNA changes |
Beam Therapeutics | BEAM-302 (Phase I/II) | Gene editing (CRISPR) | Corrects PiZ mutation in the SERPINA1 gene | One-time curative potential; direct gene repair |
BioMarin Pharma | BMN 349 (Phase I) | Small molecule | Sequesters misfolded mutant AAT | Liver-focused; reduces hepatic accumulation of toxic protein |
Krystal Biotech | KB408 (Phase I) | Gene therapy (HSV vector) | SERPINA1 gene delivery | Inhaled gene therapy; local lung targeting; avoids systemic exposure |
Summary of Competitive Insights:
Modality & Route: Emerging therapies are shifting toward SC, inhaled, and oral routes, improving patient adherence.
Mechanism: AATD pipeline is diversifying with gene/RNA editing, recombinant fusion proteins, and small molecules, moving beyond protein replacement.
Strategic Differentiators:
- Dominance of protein replacement is being challenged by next-gen approaches (RNAi, RNA editing, gene therapy).
- Takeda’s Fazirsiran and GSK/Wave’s GSK5462688 (WVE-006) could lead to next-gen molecular therapies.
- Beam and Krystal offer potential one-time curative strategies with durable outcomes.
- Inhalation and oral delivery reflect a strong trend toward improved convenience and patient compliance.
Key Companies:

Target Opportunity Profile (TOP)
While current SoC therapies effectively replace missing AAT protein and slow lung damage, they do not address the genetic root cause or liver manifestations and require burdensome IV infusions. Emerging therapies must demonstrate improved safety, disease modification, patient convenience, and durable efficacy to displace existing treatments and reshape the AATD market.
Attribute | Current Standard of Care (SoC) | Target Profile for Emerging Therapies | Key Differentiators / Gaps |
Safety | Generally well-tolerated; some infusion-related reactions | Improved safety with minimal immunogenicity and off-target effects | Reduce infusion reactions and immune responses |
Efficacy | Symptomatic relief by augmenting deficient AAT; no cure | Disease-modifying with lung protection and liver benefit | Target root cause; prevent disease progression |
Mechanism of Action | Protein replacement via plasma-derived AAT | Genetic/RNA targeting (RNAi, gene editing) or novel small molecules | Move from replacement to correction or silencing of mutation |
Route of Administration | Intravenous infusion (weekly) | Subcutaneous, inhaled, or oral for convenience | Avoid IV access and clinic visits |
Dosing Frequency | Weekly or biweekly infusions | Monthly, less frequent, or one-time administration | Reduce treatment burden and improve adherence |
Modality | Plasma-derived protein | RNAi, gene editing, fusion proteins, small molecules | Innovative modalities enabling curative or longer-lasting effects |
Durability | Requires lifelong, continuous treatment | Durable, long-lasting effect; potential for one-time cures | Significantly reduce treatment duration |
Onset of Action | Gradual increase in circulating AAT levels | Faster onset with measurable improvements | Quicker symptomatic relief and biomarker normalization |
Innovation | Established but limited innovation | First-/best-in-class, with regulatory incentives | Enhanced market appeal and faster approval |
Cost-Effectiveness | High cost due to plasma collection and infusion | Potentially lower long-term costs via durable effects | Better health economics, reduced healthcare resource use |
Why Buy Our Pharma Competitive Intelligence Report?
Our Pharma Competitive Intelligence Report is designed to give you a strategic advantage by providing deep insights into the pharmaceutical landscape. Here’s how it benefits you and your business: