Global Warm Autoimmune Hemolytic Anemia (wAIHA) Market: Industry Outlook
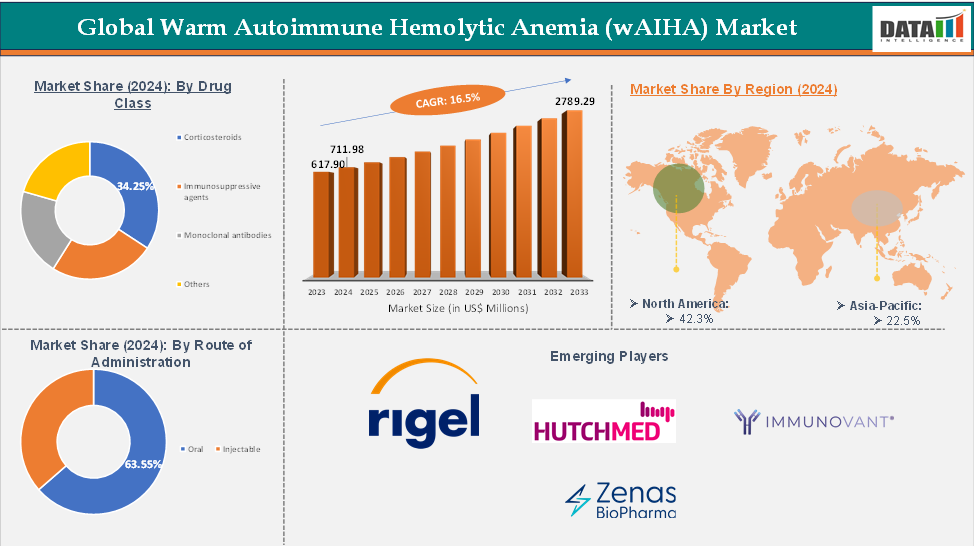
The global warm autoimmune hemolytic anemia (wAIHA) market reached US$ 617.90 Million in 2023, with a rise of US$ 711.98 Million in 2024 and is expected to reach US$ 2,789.29 Million by 2033, growing at a CAGR of 16.5% during the forecast period 2025-2033.
The global warm autoimmune hemolytic anemia (wAIHA) market is gaining attention due to increased understanding of autoimmune hematologic disorders, targeted immunotherapies, and diagnostic precision. wAIHA is the most common form of autoimmune hemolytic anemia, characterized by autoantibodies targeting red blood cells, leading to premature destruction. The market is driven by corticosteroids, immunosuppressants, biologics, and splenectomy in refractory cases.
Recent research has focused on B-cell targeting agents and complement inhibitors, with multiple clinical trials underway. Despite its rarity, regulatory incentives and increased awareness among hematologists contribute to a robust therapeutic pipeline. With ongoing innovation, improved diagnostics, and individualized treatment approaches, the wAIHA market is expected to grow steadily in the coming decade.
Global Warm Autoimmune Hemolytic Anemia (wAIHA) Market: Executive Summary
Global Warm Autoimmune Hemolytic Anemia (wAIHA) Market Dynamics: Drivers & Restraints
Driver: Increased disease awareness and early diagnosis
The wAIHA market is experiencing growth due to increased awareness of autoimmune hematological conditions among healthcare providers and hematologists. Previously, wAIHA was often misdiagnosed due to overlapping symptoms with other anemias. However, advancements in laboratory diagnostics, particularly Coombs testing, and clinical understanding of autoimmune red blood cell destruction are improving diagnosis rates. International bodies like the American Society of Hematology have provided guidelines, educational initiatives, and diagnostic algorithms, facilitating earlier identification and better patient outcomes.
For instance, in December 2024, Researchers presented a case of a patient with warm autoimmune hemolytic anemia and primary myelofibrosis, concluding that underlying myelofibrosis should be considered in patients with wAIHA and other hematologic abnormalities.
Driver: Advancements in biologic and targeted therapies
The wAIHA market is experiencing significant growth due to the development of biologic therapies and targeted immune-modulating drugs. The use of rituximab, a monoclonal antibody targeting CD20-positive B cells, has led to further research into B-cell depleting therapies, plasma cell inhibitors, and complement pathway modulators. Pipeline drugs like fostamatinib and sutimlimab are also showing promising results in clinical trials. As biotech companies invest in rare disease portfolios and regulators offer incentives for orphan indications, the biologic segment in wAIHA is expected to grow significantly.
Furthermore, advances have been achieved in complement inhibitors, neonatal Fc receptor (FcRn) monoclonal antibodies, spleen tyrosine kinase (SYK) inhibitors, and mammalian target of rapamycin (mTOR) inhibitors. This article reviews the recent advances in immunotargeted therapy for wAIHA, aiming to provide a reference for clinical practice.
Restraint: Limited epidemiological data and underdiagnosis
The market for wAIHA, a rare disease, faces challenges due to a lack of robust epidemiological data and underdiagnosis. The disease's rareness complicates investment planning, trial enrollment, and commercial forecasting. Patients often present non-specific symptoms, and specialized testing may go unrecognized without specialized testing. Limited awareness in primary care and rural healthcare systems also delays referrals to specialists. These factors hinder early intervention and broader adoption of advanced therapies, restraining market potential. Addressing this data gap through registries, surveillance studies, and awareness programs is crucial for future expansion.
Opportunity: Orphan drug development and Regulatory incentives
The wAIHA market is experiencing a surge in orphan drug development due to regulatory incentives such as market exclusivity, reduced clinical trial requirements, tax credits, and expedited review processes. These benefits lower the risk and cost of drug development, encouraging innovation in a space with high unmet need.
The orphan drug designation for agents like rituximab has paved the way for additional targeted therapies. As biopharmaceutical companies focus on niche indications with commercial potential, the wAIHA space is likely to see a surge in novel therapeutics, benefiting patients through greater access and improved outcomes.
For more details on this report, Request for Sample
Global Warm Autoimmune Hemolytic Anemia (wAIHA) Market Segment Analysis
The global warm autoimmune hemolytic anemia (wAIHA) market is segmented based on drug class, route of administration, distribution channel, and region.
Drug Class:
The corticosteroids segment from the drug class is expected to hold 34.25% of the warm autoimmune hemolytic anemia (wAIHA) markets
Corticosteroids are the primary treatment for warm autoimmune hemolytic anemia due to their rapid suppression of immune-mediated red blood cell destruction. They are prescribed immediately upon diagnosis, providing immediate hemoglobin recovery and symptom relief. Despite concerns about long-term side effects, corticosteroids are essential for initial disease control and as a bridge to second-line therapies. Their low cost, broad availability, and established clinical guidelines ensure widespread use across both developed and emerging markets.
For instance, in Decemeber 2024, Johnson & Johnson revealed findings from online abstracts and posters at the 2024 American Society of Hematology Annual Meeting on patients with warm autoimmune hemolytic anemia (wAIHA), a rare, life-threatening condition where autoantibodies cause premature red blood cell destruction. The findings highlight the significant disease burden for one in 8,000 people with wAIHA, the high unmet need for targeted therapies, and the impact on healthcare utilization.
Moreover, Healthcare providers use high-dose corticosteroids as a first-line treatment for wAIHA, an inflammatory condition, to manage symptoms by increasing hemoglobin levels and decreasing hemolysis, without an FDA- or EMA-approved therapy.
Global Warm Autoimmune Hemolytic Anemia (wAIHA) Market - Geographical Analysis
North America dominated the global warm autoimmune hemolytic anemia (wAIHA) market with the highest share of 42.3% in 2024
North America, particularly the United States, dominates the global wAIHA market due to its advanced healthcare system, early biologic therapy adoption, and strong pharmaceutical and biotech companies. The region has well-established clinical protocols for rare hematological disorders and a high density of hematology specialists. The FDA supports rare disease drug development through fast-track and orphan designations. Access to insurance and specialty care supports early diagnosis and continuity of care. Patient advocacy groups and professional societies contribute to disease awareness and funding.
For instance, in April 2025, the FDA has granted orphan drug designation to rilzabrutinib, an investigational, advanced, oral, reversible Bruton's tyrosine kinase inhibitor, for two rare diseases, warm autoimmune hemolytic anemia (wAIHA) and IgG4-related disease (IgG4-RD). This is due to the significant unmet medical need for these diseases, which have no currently approved medicine.
Asia-Pacific is the global warm autoimmune hemolytic anemia (wAIHA) market with a market share of 22.5% in 2024
The Asia-Pacific region is gaining momentum as a market for wAIHA treatment due to improved healthcare access, increased autoimmune disease awareness, and government efforts to strengthen rare disease infrastructure. Countries like Japan, China, and South Korea are seeing a rise in diagnostics and referrals in tertiary care centers. Japan's national rare disease policies facilitate diagnosis and treatment. As healthcare spending increases and hematology training expands, more patients receive appropriate care. International pharmaceutical companies are expanding clinical trial presence in Asia due to large patient populations and regulatory evolution.
For instance, in March 2025, A phase 2/3 study is underway to evaluate sovleplenib, an oral spleen tyrosine kinase inhibitor, for treating warm autoimmune hemolytic anemia. The study's results, published in The Lancet Haematology, show that sovleplenib treatment has shown an encouraging overall haemoglobin response in Chinese patients with warm autoimmune hemolytic anemia and is well tolerated.
Global Warm Autoimmune Hemolytic Anemia (wAIHA) Market – Emerging Players
The emerging players in the warm autoimmune hemolytic anemia (wAIHA) market include Rigel Pharmaceuticals, HUTCHMED, Immunovant, Inc, and Zenas BioPharma among others.
Global Warm Autoimmune Hemolytic Anemia (wAIHA) Market – Key Developments
In March 2024, HUTCHMED started the registration stage of a Phase II/III clinical trial for sovleplenib in adult patients with warm antibody autoimmune hemolytic anemia (wAIHA) in China. The trial, which has received positive data from the proof-of-concept phase and consultation with the China National Medical Products Administration, may support a future New Drug Application filing if positive. wAIHA is an autoimmune disorder with limited treatment options.
Global Warm Autoimmune Hemolytic Anemia (wAIHA) Market: Scope
Metrics | Details | |
CAGR | 16.5% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Drug Class | Corticosteroids, Immunosuppressive agents, Monoclonal antibodies, Others |
Route of Administration | Oral, Injectable | |
| Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
DMI Insights:
The global warm autoimmune hemolytic anemia (wAIHA) market, valued at US$ 711.98 Million in 2024, is expected to grow to US$ 2,789.29 Million by 2033, at a CAGR of 16.5%. This growth is driven by increased disease awareness, diagnostic advancements, and targeted biologics, particularly B-cell therapies and complement inhibitors. Corticosteroids remain the cornerstone of treatment, accounting for over 34% of the market share. North America leads the market with 42.3%, driven by strong R&D infrastructure and regulatory support. Asia-Pacific is also gaining momentum with clinical trials showing promising results. Despite underdiagnosis and limited epidemiological data, regulatory incentives, biotech investment, and emerging players are strengthening the pipeline.
The global warm autoimmune hemolytic anemia (wAIHA)market report delivers a detailed analysis with 67+ key tables, more than 55+ visually impactful figures, and 185 pages of expert insights, providing a complete view of the market landscape.