Virtual Clinical Trials Market Size
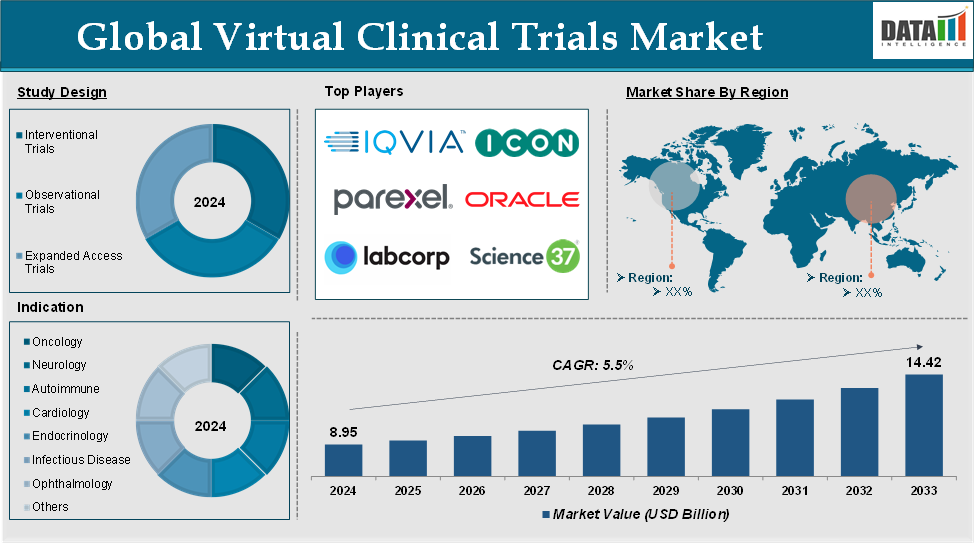
The Global Virtual Clinical Trials Market reached US$ 8.95 billion in 2024 and is expected to reach US$ 14.42 billion by 2033, growing at a CAGR of 5.5% during the forecast period 2025-2033.
In 2022, the Global Virtual Clinical Trials Market was at US$ 4.60 billion, and by 2023, it had reached US$ 4.95 billion, marking a significant growth in market value.
Virtual clinical trials refer to clinical studies that are conducted remotely, leveraging digital technologies to collect data, monitor patients and manage trial procedures without the need for participants to visit a physical clinical site. These trials, also known as decentralized clinical trials (DCTs), are designed to make the clinical trial process more accessible, flexible, and patient-centered by utilizing a variety of digital tools, mobile apps, wearables, and telemedicine platforms.
The demand for virtual clinical trials has been growing rapidly, driven by various factors that improve the efficiency, cost-effectiveness and accessibility of clinical research. Traditional clinical trials often involve physical site visits, which can be time-consuming and burdensome for patients. Virtual clinical trials remove these barriers, offering patients the convenience of participating from their homes or local healthcare facilities.
For instance, the Science 37 platform, which focuses on virtual trials, has helped improve patient participation by making it easier for patients in remote or underserved locations to join trials. They reported significant growth in recruitment rates and participant retention due to the ability to reach broader patient populations.
Executive Summary
For more details on this report – Request for Sample
Virtual Clinical Trials Market Dynamics: Drivers & Restraints
Improved Data Accuracy and Real-Time Monitoring
The improved data accuracy and real-time monitoring are significantly driving the growth of the virtual clinical trials market and are expected to drive the market over the forecast period. Virtual clinical trials enable continuous and real-time data monitoring, such as vital signs, symptoms and medication adherence. This allows for more accurate tracking of patient health throughout the trial without delays or discrepancies that might occur with periodic in-person visits.
For instance, in Medable's platform, patients are equipped with wearables that monitor physiological data such as heart rate, oxygen levels, and physical activity. This data is transmitted to researchers in real time, enabling early detection of adverse events and faster decision-making. This level of monitoring helps ensure that data is both accurate and timely, reducing the reliance on self-reporting or retrospective data collection, which can be prone to errors.
Real-time monitoring fosters patient engagement by providing instant feedback to participants on their progress. This increases adherence to the trial protocol, especially in long-duration studies, as patients can be reminded to take medication or perform necessary activities through digital tools. For instance, in June 2024, Materna Medical launched a groundbreaking virtual clinical trial evaluating the treatment of vaginismus symptoms using the Milli Vaginal Dilator. This groundbreaking clinical investigation aims to gather valuable data on the effectiveness of the Milli Vaginal Dilator to help relieve the symptoms of vaginismus and related painful intercourse.
Data Privacy and Security Concerns
Data privacy and security concerns are significant challenges that can hamper the growth of the virtual clinical trials market. Since virtual clinical trials rely heavily on the collection, storage and transmission of sensitive patient data such as medical histories, personal identifiers and health metrics, there is an inherent risk related to data breaches, unauthorized access and non-compliance with data protection regulations.
Virtual clinical trials depend on digital platforms, wearables, and mobile applications that collect and transmit sensitive patient information. If these systems are not properly secured, they become vulnerable to cyberattacks, data breaches, or unauthorized access. For instance, a Colorado-based pathology laboratory is notifying more than 1.8 million patients that their sensitive information was compromised one of the largest breaches reported by a medical testing lab to US federal regulators, making the healthcare industry especially vulnerable to hackers.
Patients are often hesitant to share sensitive personal health data through digital platforms due to concerns over privacy and the potential misuse of their information. This mistrust can lead to lower enrollment rates and reduced participation in virtual trials, hindering the effectiveness of such trials. For instance, according to the HHS Cybersecurity Program, 630+ total healthcare organizational breaches and 29 million healthcare records were breached. This hesitation impacts recruitment and retention rates, which are essential to the success of any decentralized clinical trials.
Virtual Clinical Trials Market Segment Analysis
The global virtual clinical trials market is segmented based on study design, indication, phase and region.
By Indication:
The oncology segment is expected to dominate the virtual clinical trials market share
The oncology segment grew from US$ 1.30 billion in 2022 to US$ 1.42 billion in 2023, owing to rising adoption in the global market.
Oncology trials often require large, diverse patient populations due to the complexity and variety of cancers. Virtual trials make it easier to recruit patients from a wide geographic area, particularly those living in remote regions or underserved areas where cancer treatment options are limited. For instance, in October 2024, Medable announced a partnership with technology giant Google Cloud to bring the company’s digital, decentralized clinical trial platform to the Google Cloud Marketplace. Medable’s system was selected by the Nova Scotia Health Innovation Hub to improve care accessibility for remote oncology patients in rural Canada.
Oncology trials often have long durations, with patients requiring frequent monitoring, assessments and data collection. Virtual clinical trials can streamline these processes by using remote patient monitoring tools and wearables to track health metrics in real time, reducing the need for patients to visit the trial site repeatedly.
For instance, a major Novartis oncology trial for a new treatment in chronic lymphocytic leukemia (CLL) used wearables and remote monitoring tools to track patient responses, side effects and overall health. This approach minimized the need for frequent in-person visits, enhancing patient compliance and reducing the logistical burden on both patients and researchers.
Virtual Clinical Trials Market Geographical Analysis
North America is expected to hold a significant position in the virtual clinical trials market share
North America led the Virtual Clinical Trials Market in 2022 with a market size of US$ 2.23 billion and expanded further to US$ 2.40 billion in 2023.
North America especially the United States and Canada has a highly developed healthcare technology ecosystem, with cutting-edge digital health tools, wearables, telemedicine platforms, and electronic data capture systems readily available to support virtual clinical trials. The availability of advanced digital technologies and platforms is one of the key drivers of the region's dominance in this market.
For instance, in November 2024, Medable Inc. announced Medable AI – generative AI capabilities that help sponsors and clinical research organizations build digital and decentralized trials faster with complete visibility and control over technology setup. Medable is the first to incorporate generative AI in the study-build process, ultimately driving the industry to a breakthrough, one-day study startup.
North America is home to some of the largest pharmaceutical and biotechnology companies globally, many of which are at the forefront of adopting virtual trial methodologies to streamline their research and development processes. These companies are keen to reduce trial costs, improve patient recruitment, and enhance data quality through virtual trial solutions.
For instance, Pfizer, Johnson & Johnson and Eli Lilly, among other major companies, have run multiple virtual trials in North America. For example, Pfizer conducted a COVID-19 vaccine trial that incorporated virtual trial methods to enable global patient recruitment and data collection. This highlights the region's leadership in adopting virtual clinical trial models.
Asia-Pacific is growing at the fastest pace in the virtual clinical trials market
Asia-Pacific recorded strong growth, increasing from US$ 0.78 billion in 2022 to US$ 0.85 billion in 2023, supported by rising investments and growing demand in emerging economies like China and India.
The adoption of digital health solutions, including telemedicine and mobile health apps has been growing rapidly in the Asia-Pacific region. The COVID-19 pandemic accelerated the use of telehealth services, which laid the foundation for the adoption of virtual clinical trials. This digital transformation has made it easier for healthcare providers to integrate remote monitoring, patient engagement tools, and digital consent processes into clinical trials.
For instance, China, with its fast-growing digital infrastructure, has been a leader in integrating telemedicine and virtual healthcare solutions into clinical trials. For instance, Shanghai-based company Wuxi AppTec facilitated virtual trials for oncology research, using telemedicine, mobile health monitoring, and eConsent to connect patients in remote areas with researchers, improving patient access and trial participation.
Governments and regulatory bodies in APAC are increasingly supportive of decentralized and virtual clinical trials, as they recognize the need for faster, more efficient drug development processes. Countries like Japan, India, and Singapore have begun to introduce and streamline regulations that facilitate the use of digital health technologies and virtual trials.
For instance, in Japan, the Pharmaceutical and Medical Devices Agency (PMDA) has been actively working on guidelines to encourage the use of remote monitoring, telehealth consultations, and mobile devices for clinical trials. Similarly, in India, the Central Drugs Standard Control Organization (CDSCO) has provided guidelines to facilitate the use of digital tools in clinical research, further encouraging virtual trials.
Virtual Clinical Trials Market Major Players
The major global players in the virtual clinical trials market include IQVIA, ICON plc, Laboratory Corporation of America Holdings, Science 37, Parexel International Corporation, Oracle Corporation, Medidata Solutions, Signant Health, Veristat, LLC, Sanofi and among others.
Scope
| Metrics | Details | |
| CAGR | 5.5% | |
| Market Size Available for Years | 2018-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Volume (Units) | ||
| Segments Covered | Study Design | Interventional Trials, Observational Trials and Expanded Access Trials |
| Indication | Oncology, Neurology, Autoimmune/Inflammation, Cardiovascular Disease, Metabolic/Endocrinology, Infectious Disease, Ophthalmology and Others | |
| Phase | Phase I, Phase II, Phase III and Phase IV | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials, product pipelines, and forecasts upcoming advancements in medical devices and pharmaceuticals.
- Product Performance & Market Positioning: Analyzes product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: Covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyzes competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient product delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global virtual clinical trials market report delivers a detailed analysis with 62 key tables, more than 58 visually impactful figures, and 126 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2025
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.