Global Toxoid Vaccine Market – Industry Trends & Outlook
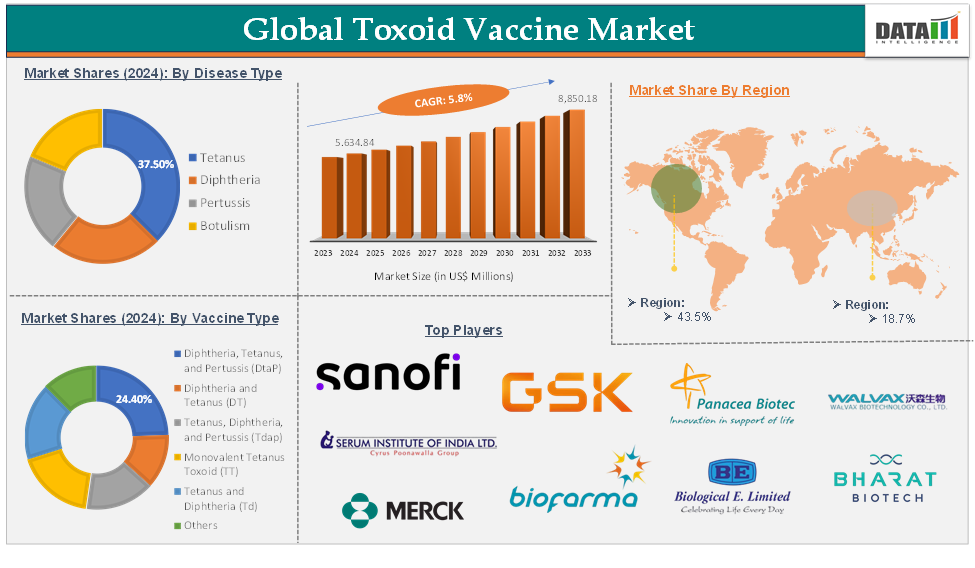
The global toxoid vaccine market reached US$ 5,634.84 Million in 2024 and is expected to reach US$ 8,850.18 Million by 2033, growing at a CAGR of 5.8 % during the forecast period of 2025-2033.
The global toxoid vaccine market is characterized by the production and distribution of vaccines derived from inactivated bacterial toxins, or toxoids, which play a crucial role in preventing diseases such as tetanus and diphtheria. These vaccines are particularly important in pediatric and maternal immunization programs and are administered widely through hospitals, clinics, and vaccination centers.
Key drivers of market growth include the rising incidence of vaccine-preventable diseases and the expansion of government-led immunization initiatives. Technological advancements in vaccine development, such as improved safety and efficacy profiles and the emergence of combination vaccines, are also significant contributors to the market’s expansion.
Current trends in the toxoid vaccine market include the dominance of the tetanus segment, which accounts for the largest market share due to the continued risk of tetanus-related complications in unvaccinated and high-risk populations. North America leads the market, driven by advanced healthcare infrastructure and high immunization coverage, while the Asia-Pacific region is expected to experience the fastest growth, fueled by rising healthcare investments, higher birth rates, and increased government focus on immunization.
Opportunities in the market are abundant, particularly in emerging economies where expanding healthcare access and government support for vaccination are accelerating growth. The development of new and improved toxoid vaccines, the adoption of combination vaccines, and ongoing research and development efforts present further avenues for market expansion.
Global Toxoid Vaccine Market – Executive Summary
Global Toxoid Vaccine Market Dynamics: Drivers
Rising prevalence of vaccine-preventable diseases
The rising prevalence of vaccine-preventable diseases is a key factor driving the growth of the global toxoid vaccine market. In recent years, outbreaks of diseases such as tetanus and diphtheria have become more frequent, particularly in regions with gaps in immunization coverage or where vaccination programs have been disrupted.
As more people become susceptible to these preventable diseases, the demand for effective vaccines, especially toxoid vaccines, has grown significantly. Governments and health organizations are responding by expanding immunization initiatives and investing in public awareness campaigns to encourage vaccination.
According to ScienceDirect Vaccines data from April 2025, diphtheria-tetanus-pertussis-containing vaccines (DTPCVs) are at the forefront of global combination vaccine innovation. However, the approval and accessibility of high-valent DTPCVs vary internationally due to differences in regulatory requirements. In response to pressing public health needs, certain national regulatory authorities (NRAs) have adopted more flexible and accelerated processes to speed up the licensing and introduction of these critical vaccines.
Additionally, the growing adoption of combination vaccines, which protect against multiple diseases with a single shot, is helping to address the challenge of rising disease prevalence. These combination vaccines are especially valuable in areas with limited access to healthcare, as they simplify immunization schedules and improve overall coverage. As a result, the market for toxoid vaccines is expected to continue its robust growth trajectory, driven by the urgent need to control and prevent vaccine-preventable diseases worldwide.
Expansion of adult and booster vaccination programs
The expansion of adult and booster vaccination programs is a significant driver for the global toxoid vaccine market. Traditionally, vaccination efforts have focused on children, but there is growing recognition that adults also require ongoing protection against infectious diseases.
In response, many countries are broadening their immunization schedules to include routine adult and booster vaccinations, supported by government campaigns and public health initiatives. For example, health authorities in North America and Europe have established comprehensive adult vaccination programs, while developing regions are rapidly building their capacity to improve adult vaccine coverage.
For instance, in October 2024, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) voted to expand its recommendation for the use of certain pneumococcal vaccines, including Pfizer’s PREVNAR 20 (20-valent Pneumococcal Conjugate Vaccine), to all adults aged 50 and older.
This recommendation is pending final approval by the CDC Director and the Department of Health and Human Services. As a result, demand for adult and booster vaccines, including toxoid vaccines, continues to rise, driving innovation and investment in new vaccine formulations and delivery strategies.
Global Toxoid Vaccine Market Dynamics: Restraints
High cost of vaccines
The high cost of vaccines is a significant restraint for the global toxoid vaccine market. Developing, manufacturing, and distributing toxoid vaccines require substantial financial investment, particularly due to the complex production processes and stringent regulatory requirements involved.
These processes often necessitate advanced technology, skilled labor, and robust quality control systems, all of which contribute to elevated operational expenses. Additionally, maintaining a reliable cold chain for vaccine storage and transportation further increases costs, especially in remote or resource-limited regions. Even with support from international organizations, the financial burden can restrict the scale of vaccination programs, undermining efforts to control and prevent diseases like tetanus and diphtheria.
Furthermore, the high cost of research and development for new or improved toxoid vaccines can deter smaller manufacturers from entering the market, reducing competition and innovation. This can lead to slower adoption of new technologies and limit the introduction of more affordable or effective vaccine options. Overall, the high cost of vaccines remains a critical challenge, constraining market growth and impeding global public health goals.
Global Toxoid Vaccine Market Dynamics: Opportunities
Expansion in emerging markets
The expansion in emerging markets presents a major opportunity for the global toxoid vaccine market. Rapidly growing economies in regions such as Asia-Pacific, Latin America, and parts of Africa are witnessing significant improvements in healthcare infrastructure, increased government spending on immunization, and greater public awareness of the importance of vaccines.
Countries like India and China have launched large-scale immunization campaigns targeting both urban and rural populations, leading to a substantial rise in vaccine accessibility and coverage. International organizations such as the WHO and UNICEF are also playing a crucial role by providing funding, technical support, and logistical assistance to strengthen vaccination programs in these regions.
Overall, the ongoing expansion in emerging markets not only increases the reach of toxoid vaccines but also drives innovation and competition, making it a key growth engine for the global toxoid vaccine market.
For more details on this report, Request for Sample
Global Toxoid Vaccine Market - Segment Analysis
The global toxoid vaccine market is segmented based on disease type, vaccine type, age group, end-user, and region.
Disease Type:
The tetanus disease type segment is expected to hold 37.5% of the global toxoid vaccine market in 2024
The tetanus disease type segment is a vital part of the global toxoid vaccine market, as it focuses on preventing various forms of tetanus, a potentially life-threatening condition caused by the bacterium Clostridium tetani. Tetanus occurs when bacterial spores enter the body through wounds or cuts, producing a powerful neurotoxin that leads to severe muscle stiffness and spasms.
One of the primary drivers is the increasing awareness about the severe consequences of tetanus infections, which has led to intensified global immunization programs, particularly in regions with high infection rates and limited healthcare access. Government initiatives and public health campaigns play a critical role, as they promote vaccination awareness and drive higher coverage rates, especially in developing nations where the burden of tetanus remains significant.
Another important driver is the development and adoption of combination vaccines, such as DTaP and Tdap, which protect against diphtheria, tetanus, and pertussis in a single shot. These combination vaccines simplify immunization schedules, reduce the number of required injections, and increase compliance, thereby boosting overall vaccination rates.
For instance, in April 2024, Union Health Secretary Shri Apurva Chandra visited the manufacturing facility of the global pharmaceutical company Bilthoven Biologicals in Utrecht, Netherlands. A comprehensive presentation was given on the company’s future manufacturing plans. Bilthoven Biologicals B.V. specializes in producing pharmaceutical products, including vaccines for polio, diphtheria-tetanus-polio, and tetanus, as well as the bacillus Calmette-Guérin (BCG) vaccine. These factors have solidified the segment's position in the global toxoid vaccine market.
Global Toxoid Vaccine Market – Geographical Analysis
North America is expected to hold 43.5% of the global toxoid vaccine market in 2024
North America holds the largest share of the global toxoid vaccine market. This dominant position is driven by several key factors. The region benefits from advanced healthcare infrastructure and mature immunization coverage systems, which ensure high vaccine uptake and efficient distribution. Robust government support, including sustained legislative and funding initiatives, underpins widespread vaccination programs and enables the rapid adoption of new vaccine recommendations.
Continuous advancements in vaccine development and manufacturing, along with significant investments in research and development by leading pharmaceutical companies, further strengthen North America’s market position. The presence of major vaccine manufacturers and a strong focus on innovation have led to the introduction of high-precision and combination vaccines, such as DTaP and Tdap, which simplify immunization schedules and increase coverage. High awareness of vaccine-preventable diseases, coupled with proactive public health campaigns, drives demand and ensures high vaccination rates across all age groups.
For instance, in June 2024, the U.S. Food and Drug Administration (FDA) approved CAPVAXIVE (Pneumococcal 21-valent Conjugate Vaccine, also known as V116) for use in adults aged 18 years and older. This vaccine is indicated for the prevention of invasive pneumococcal disease (IPD) and pneumococcal pneumonia caused by Streptococcus pneumoniae in adults. Thus, the above factors are consolidating the region's position as a dominant force in the global toxoid vaccine market.
Asia Pacific is expected to hold 18.7% of the global toxoid vaccine market in 2024
Vaccination remains one of the most cost-effective strategies for safeguarding children’s health and securing their futures. India’s Universal Immunization Programme (UIP), recognized as one of the largest immunization initiatives globally, has played a crucial role in lowering mortality rates and controlling infectious diseases. Each year, the UIP reaches approximately 29 million pregnant women and 27 million newborns.
As per the Ministry of Health and Family Welfare data in November 2024, through the UIP, every child receives 27 vaccine doses from birth up to 16 years of age, protecting against 12 vaccine-preventable diseases. Additionally, every pregnant woman is administered two doses of the Td (Tetanus and Adult Diphtheria) vaccine.
The region has seen significant government investment in national immunization programs, with countries like India and China playing central roles. India supplies 60% of vaccines for global immunization initiatives, and China covers 90% of its domestic vaccine needs through robust manufacturing capacity.
The adoption of higher-valent combination vaccines, such as pentavalent and hexavalent formulations, is increasing, which streamlines immunization schedules and improves coverage rates. Third, funding and procurement support from international organizations like UNICEF, Gavi, and the World Health Organization (WHO) are sustaining vaccine accessibility, particularly in low- and middle-income countries across South Asia and Southeast Asia. Thus, the above factors are consolidating the region's position as a dominant force in the global toxoid vaccine market.
Global Toxoid Vaccine Market – Competitive Landscape
The major global players in the toxoid vaccine market include Sanofi, GSK plc, Merck/Sanofi Pasteur (MSP), Serum Institute of India Pvt. Ltd., Bio Farma, Biological E, Panacea Biotec, Walvax Biotechnology Co., Ltd., Bharat Biotech., MassBiologics, and Mitsubishi Tanabe Pharma Corporation among others.
Global Toxoid Vaccine Market – Key Developments
In December 2024, LimmaTech Biologics AG, a clinical-stage biotech company, announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track designation to its multivalent toxoid vaccine candidate, LBT-SA7. This vaccine is being developed to prevent skin and soft tissue infections (SSTIs) caused by the bacterial pathogen Staphylococcus aureus (S. aureus).
Global Toxoid Vaccine Market – Scope
Metrics | Details | |
CAGR | 5.8% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Disease Type | Tetanus, Diphtheria, Pertussis, Botulism |
Vaccine Type | Diphtheria, Tetanus, and Pertussis (DtaP), Diphtheria and Tetanus (DT), Tetanus, Diphtheria, and Pertussis (Tdap), Monovalent Tetanus Toxoid (TT), Tetanus and Diphtheria (Td), Others | |
Age Group | Neonates, Children, Adults, Others | |
End-User | Hospitals and Clinics, Government Organizations, Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global toxoid vaccine market report delivers a detailed analysis with 73 key tables, more than 68 visually impactful figures, and 173 pages of expert insights, providing a complete view of the market landscape.