Pediatric Vaccine Market Size
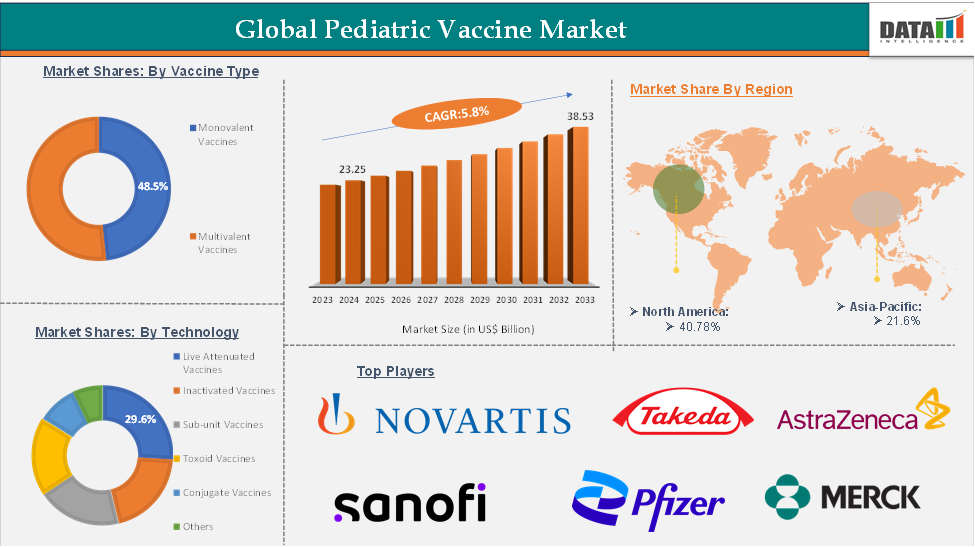
The global pediatric vaccine market size reached US$ 23.25 Billion in 2024 and is expected to reach US$ 38.53 Billion by 2033, growing at a CAGR of 5.8% during the forecast period 2025-2033.
Pediatric Vaccine Market Overview
The global pediatric vaccine market is experiencing robust growth, driven by rising awareness of childhood immunization, increasing government initiatives, and ongoing technological advancements in vaccine development. Key factors fueling demand include the growing prevalence of infectious and chronic diseases among children, the introduction of combination and multivalent vaccines, and enhanced R&D efforts by major pharmaceutical companies.
North America currently leads with the largest market share, while the Asia-Pacific region is expected to see significant expansion due to broader vaccination coverage and higher disposable incomes.
Executive Summary
For more details on this report – Request for Sample
Pediatric Vaccine Market Dynamics: Drivers & Restraints
Rising prevalence of infectious diseases is significantly driving the pediatric vaccine market growth
The increasing prevalence of infectious diseases is a major factor driving growth in the pediatric vaccine market, as outbreaks of measles, mumps, rubella, and pertussis continue to underscore the importance of immunization. For instance, Rubella remains the leading vaccine-preventable cause of birth defects worldwide, with an estimated 100,000 infants born each year with congenital rubella syndrome (CRS). Infection with the rubella virus during pregnancy can lead to miscarriage, fetal death, or CRS, with the most severe outcomes occurring when infection happens in the first trimester.
Despite the availability of a safe and cost-effective vaccine, rubella continues to pose a significant public health challenge; in 2022, there were an estimated 17,865 reported cases across 78 countries. This underscores the urgent need for broader vaccine coverage and the introduction of rubella-containing vaccines in countries where they are not yet part of routine immunization programs.
Governments and healthcare organizations are ramping up vaccination efforts to curb transmission, leading to higher adoption rates. Additionally, rising awareness among parents and caregivers about the critical role of vaccines in disease prevention is further accelerating market expansion.
The high treatment cost is hampering the growth of the pediatric vaccine market
The high cost of vaccine development and distribution poses a significant challenge to the pediatric vaccine market, limiting accessibility and slowing adoption rates. The complex research, clinical trials, and regulatory approvals required for vaccine development demand substantial financial investment, making it difficult for manufacturers to introduce new vaccines at affordable prices.
Pediatric Vaccine Market, Segment Analysis
The global pediatric vaccine market is segmented based on disease type, vaccine type, technology, and region.
The multivalent vaccines from the vaccine type segment is expected to hold 51.5% of the market share in 2024 in the pediatric vaccine market
The multivalent vaccines segment is expected to lead the pediatric vaccine market, primarily because these vaccines offer protection against multiple diseases with a single injection, a feature highly valued by healthcare professionals and parents for its convenience and efficiency. This approach simplifies immunization schedules, increases compliance, and improves vaccine coverage, especially in areas with high disease prevalence or limited healthcare access.
Product innovation is a significant driver in this segment, with ongoing advancements in vaccine formulations, such as the development of safer and more effective conjugate and recombinant vaccines, expanding the reach and effectiveness of multivalent options. Pharmaceutical companies are investing heavily in research and development to create new and improved multivalent vaccines, further accelerating market growth. For instance, in November 2024, Abbott India announced the launch of its new pneumococcal conjugate vaccine, PneumoShield 14, in India. Designed for children as young as 6 weeks old, PneumoShield 14 provides broader protection compared to existing vaccines currently available in the country.
Additionally, strong support from government and international immunization programs is helping to promote the adoption of multivalent vaccines, particularly in regions where comprehensive disease prevention is a public health priority
Pediatric Vaccine Market, Geographical Analysis
North America is expected to dominate the global pediatric vaccine market with a 39.7% share in 2024
North America is projected to maintain its dominance in the pediatric vaccine market, supported by a highly advanced healthcare infrastructure, comprehensive immunization programs, and strong government mandates that ensure widespread vaccine coverage among children. For instance, in May 2025, the U.S. Department of Health and Human Services (HHS) and the National Institutes of Health (NIH) have announced the development of a next-generation universal vaccine platform called Generation Gold Standard. This innovative platform utilizes a beta-propiolactone (BPL)-inactivated, whole-virus approach to advance vaccine technology.
The region’s economic strength enables significant investments in research and development, fostering continuous innovation and rapid adoption of new vaccine technologies.
Additionally, the presence of leading vaccine manufacturers such as Pfizer, Moderna, and Merck ensures a reliable and timely vaccine supply. Public awareness and acceptance of regular childhood immunizations remain high, further contributing to robust vaccination rates and sustained market leadership for North America in the global pediatric vaccine sector.
Asia-Pacific is growing at the fastest pace in the pediatric vaccine market, holding 20.8% of the market share
The Asia-Pacific region is emerging as the fastest-growing market for pediatric vaccines, driven by several important factors. A large and youthful population creates high demand for immunization, while increasing public awareness and strong government initiatives are broadening vaccination coverage. Countries such as India and China are making notable progress in enhancing healthcare infrastructure and affordability, making vaccines more accessible to families across the region.
Furthermore, continuous investments from both public and private sectors, advancements in vaccine technology, and active participation from leading manufacturers are boosting the region’s capacity to address infectious diseases.
Pediatric Vaccine Market Competitive Landscape
Top companies in the pediatric vaccine market include Pfizer Inc., Sanofi, Merck & Co., Takeda Pharmaceutical Company Limited, Indian Immunological Limited, Bharat Biotech, Panacea Biotec, Sinovac Biotech, AstraZeneca, Novartis AG, among others.
Pediatric Vaccine Market, Key Developments
In July 2024, GSK has unveiled its new multichannel campaign, ‘Ab India Banega 7-Star,’ aimed at encouraging parents to protect their children’s future through seven essential vaccinations that guard against 14 serious diseases. These include chickenpox, hepatitis A, hepatitis B, meningitis, measles, mumps, rubella, pneumonia, influenza, diphtheria, tetanus, pertussis, Hib infection, and polio. The campaign highlights the importance of comprehensive immunization for children’s long-term health and well-being.
Pediatric Vaccine Market Scope
Metrics | Details | |
CAGR | 5.8% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Disease Type | Infectious Diseases, Allergies |
Vaccine Type | Monovalent Vaccines, Multivalent Vaccines | |
Technology | Live Attenuated Vaccines, Inactivated Vaccines, Sub-unit Vaccines, Toxoid Vaccines, Conjugate Vaccines, Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global Pediatric Vaccine market report delivers a detailed analysis with 60+ key tables, more than 55+ visually impactful figures, and 178 pages of expert insights, providing a complete view of the market landscape.