Pyelonephritis Treatment Market Overview
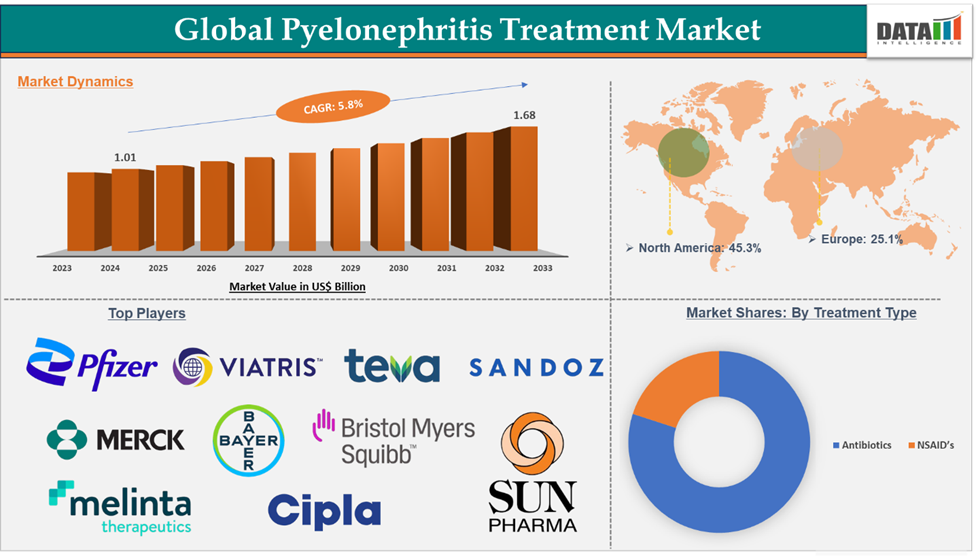
Pyelonephritis Treatment Market reached US$ 1,014.39 million in 2024 and is expected to reach US$ 1,682.83 billion by 2033, growing at a CAGR of 5.8% during the forecast period 2025-2033.
The pyelonephritis treatment market is experiencing growth due to the rising prevalence, increasing research and development activities, launch of advanced combination antibiotics for the treatment. However, the antimicrobial resistance, side effects associated with the drugs, and the high cost of chronic pyelonephritis treatment may restrain the market growth.
Pyelonephritis is a bacterial kidney infection, usually caused by ascending UTI (urinary tract infection), rarely by hematogenous spread. Most of the patient population gets an ascending infection, and the hematogenous spread is limited to highly immunocompromised patients with ureteral obstructions. In the ascending infection, the pathogens first reach the bladder and then spread to the kidneys. In the hematogenous spread, the pathogens infect the kidneys by traveling through the bloodstream. The common symptoms include fever, dysuria, costovertebral angle pain (flank pain), etc.
Executive Summary
For more details on this report – Request for Sample
Pyelonephritis Treatment Market Dynamics: Drivers & Restraints
The rising prevalence of pyelonephritis is driving the market growth
Pyelonephritis, also known as kidney infection, is a major complication of ascending urinary tract infections. Several population groups are at a higher risk of developing pyelonephritis, and they include the elderly, pregnant women, kidney transplant patients, Immuno-compromised patients, patients with recurrent UTIs, and patients with uncontrolled diabetes.
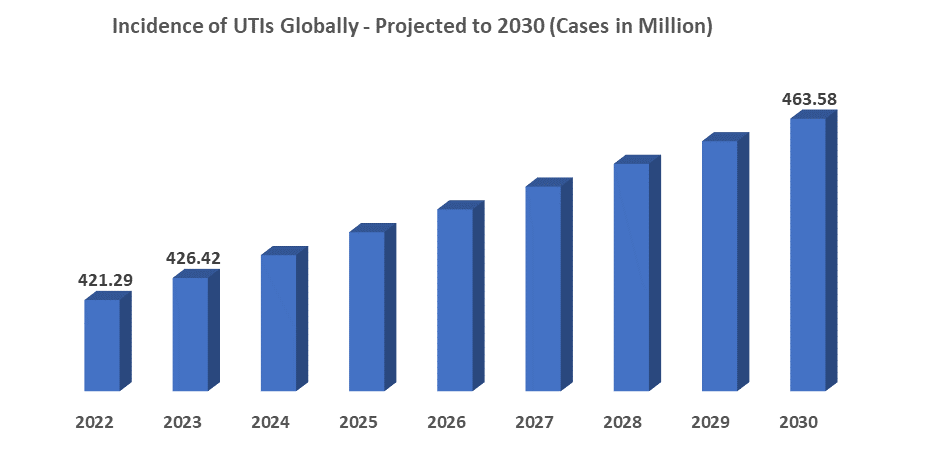
For instance, a global epidemiology study on urinary tract infection has stated that, in 2019, nearly 404.6 million people worldwide had UTI, which is a significant increase in recent times. As per dataM estimates, by 2023, this population had reached 426.42 million and is projected to reach 463.57 million by 2030.
Moreover, diabetic patients, especially women with type 2 diabetes, are at a higher risk of developing pyelonephritis. Diabetes causes metabolic changes in these patients, and once they contract any UTI, they become highly susceptible to acute pyelonephritis. The aging population and rising prevalence of diabetes put more population at higher risk of developing pyelonephritis.
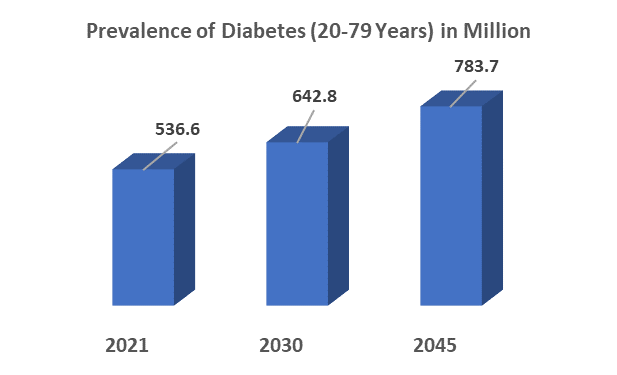
For instance, according to the International Diabetes Federation statistics, there are approximately 536 million diabetic population (20 to 79 years) worldwide, and this number is anticipated to reach 642 million by 2030, and 743 million by 2045.
As the prevalence of these diseases rises, the incidence of pyelonephritis is anticipated to increase, creating the demand for advanced therapeutics and driving the pyelonephritis treatment market.
Rising antimicrobial resistance may restrain the market growth
The rising antimicrobial resistance is the major restraint for the market growth, and for the majority of the antibiotics currently prescribed, the pathogens are already becoming resistant. This rapid growth in antimicrobial resistance may limit the usage of antibiotics and may stunt market growth in the forecast period.
Pyelonephritis Treatment Market Segment Analysis
The global pyelonephritis treatment market is segmented based on type, treatment type, route of administration, and region.
Antibiotics in the treatment type segment accounted for 84.05% of the market share in 2024 in the global pyelonephritis treatment market.
Pyelonephritis is a bacterial infection of kidney tissues, and antibiotics are the mainstay of treatment for the condition. They help to eliminate the causative infection and alleviate the condition. As per several research studies, in 90% of pyelonephritis cases, Escherichia coli is the main pathogen. This pathogen is susceptible to antibiotics such as fluoroquinolones, cephalosporins, or sulfamethoxazole-trimethoprim, and responds well to these drugs.
Among the antibiotics group, fluoroquinolones such as ciprofloxacin, cephalosporins such as cephalexin, and sulfamethoxazole-trimethoprim combinations are widely prescribed due to their relatively low antimicrobial resistance. Several health agencies have recommended these antibiotics to be used as first-line agents, both in empirical therapy and after diagnostic confirmation. For instance, according to National Institute for Health and Care Excellence (NICE) guidelines, ciprofloxacin, cefalexin, and other cephalosporin drugs are prescribed as first-line agents for the treatment of acute pyelonephritis. Moreover, according to the European Association of Urology, fluoroquinolones and cephalosporins are the only antimicrobial agents that can be recommended for oral empirical treatment of uncomplicated pyelonephritis.
Moreover, pregnant women are at a higher risk of developing pyelonephritis. Several antibiotics are considered to be safe and effective to use during pregnancy and these include some cephalosporins and fluoroquinolones. This reflects the therapeutic importance of antibiotics in managing pyelonephritis and their higher adoption rate and higher market share.
Pyelonephritis Treatment Market Geographical Analysis
North America dominated the pyelonephritis treatment market with the highest share of 45.3% in 2024
North America dominates the global pyelonephritis treatment market due to its advanced healthcare services, easy accessibility to a variety of treatments, availability of branded drugs offered by global pharmaceutical industry leaders, etc.
The United States is well known for its advanced healthcare industry. The higher per-capita income, expenditure, and investments in the healthcare industry and stringent yet favorable regulatory policies attract the manufacturers to develop novel therapies and launch them first in the country. In recent times, the R&D activities concerned with complicated UTIs and subsequent regulatory approvals have risen significantly.
For instance, in February 2024, the U.S. Food and Drug Administration approved EXBLIFEP, developed by Allecra Therapeutics for the treatment of complicated urinary tract infections (cUTIs), including pyelonephritis, in patients 18 years and older. EXBLIFEP is a combination of cefepime and enmetazobactam. This novel drug will be made available first in the U.S. market.
Moreover, GSK plc is planning to file for regulatory approval in the U.S. for tebipenem by 2H of 2025. Tebipenem is being developed by Spero Therapeutics for the treatment of cUTIs, and under the strategic partnership, GSK has acquired commercial rights for the drug. The Drug is anticipated to be launched first in the U.S. market by the end of 2025.
Hence, considering these factors, along with the epidemiological data and sales figures of other product categories by all market players, North America is designated as a dominant region in the global market.
Pyelonephritis Treatment Market Major Players
The major players in the pyelonephritis treatment market are Pfizer Inc., Teva Pharmaceuticals USA, Inc., Bayer AG, Sandoz AG, Bristol-Myers Squibb Company, Viatris Inc., Melinta Therapeutics, LLC., Cipla, Merck & Co., Inc., and Sun Pharmaceutical Industries Ltd., among others.
Key Development
In April 2025, Alnylam Pharmaceuticals, Inc. announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) had adopted a positive opinion on recommending approval of vutrisiran for the treatment of wild-type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy (ATTR-CM).
In March 2025, the U.S. Food and Drug Administration (FDA) approved the 7th siRNA therapy fitusiran (Qfitlia) developed by Alnylam Pharmaceuticals, Inc. for the treatment of Hemophilia A or B. With this approval, fitusiran has become the first siRNA therapy to get approval in the hemophilia landscape, and it works by lowering antithrombin (AT), a protein that inhibits blood clotting.
Market Scope
Metrics | Details | |
CAGR | 5.8% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Type | Acute Pyelonephritis and Chronic Pyelonephritis |
Treatment Type | Antibiotics and NSAIDs | |
Route of Administration | Oral and Parenteral | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |