Pulse Oximeter Market – Industry Outlook & Overview
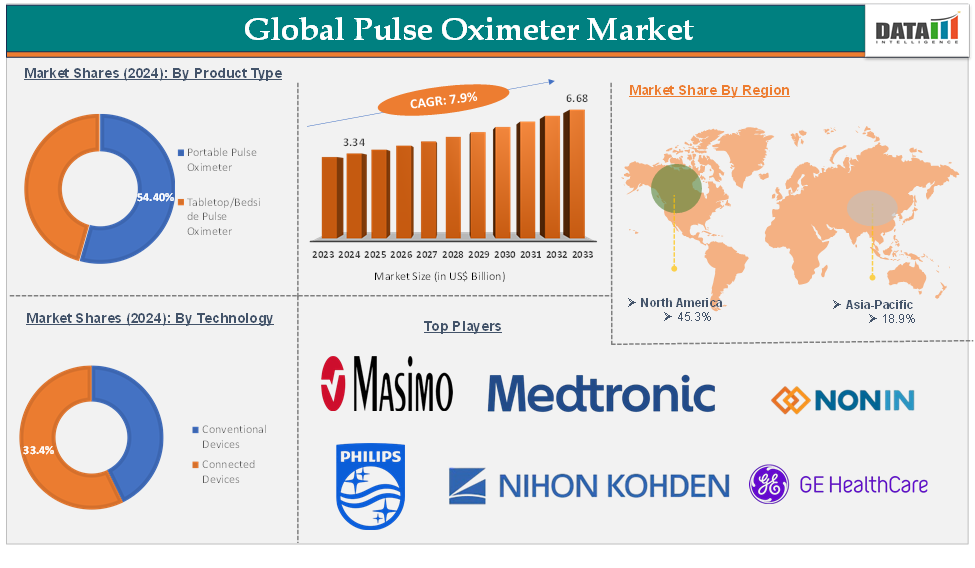
Pulse Oximeter Market reached US$ 3.34 Billion in 2024 and is expected to reach US$ 6.68 Billion by 2033, growing at a CAGR of 7.9 % during the forecast period 2025-2033.
A pulse oximeter is a non-invasive medical device that measures the oxygen saturation (SpO2) of a person's blood and their pulse rate, typically using light beams through a sensor placed on a fingertip or earlobe. It provides real-time information about how well oxygen is being delivered throughout the body and is widely used in hospitals, clinics, and increasingly in home healthcare settings.
The global pulse oximeter market is being driven by several key factors, including the rising prevalence of respiratory diseases, an aging population that is more susceptible to chronic illnesses, increase in surgical procedures and hospitalizations, technological advancements that have improved device accuracy and usability, and greater awareness about the importance of monitoring blood oxygen levels.
Significant opportunities exist in the expansion of pulse oximeter use in emerging economies, where rising healthcare investment and increased disease awareness are fueling demand. The growing adoption of wearable and portable pulse oximeters for home and remote patient monitoring, integration with smartphone applications, and the development of AI-powered devices for better health outcomes are also creating new avenues for growth. Strategic collaborations, R&D investments, and product innovation further enhance market prospects.
Key trends shaping the pulse oximeter market include the miniaturization and increased portability of devices, making them more accessible for home and fitness use. There is a surge in demand for wearable oximeters and connected devices that offer real-time data tracking and sharing with healthcare providers. The market is also seeing a shift toward automated, wireless, and remote monitoring solutions, as well as increased use in fitness and wellness for athletes and health-conscious consumers. Manufacturers are focusing on improving accuracy, user experience, and affordability, further driving market adoption.
Executive Summary
For more details on this report – Request for Sample
Pulse Oximeter Market Dynamics: Drivers
Technological advancements in pulse oximeters
Technological advancements in pulse oximeters are significantly driving the global pulse oximeter market's growth, which is expected to continue throughout the market forecast period. These innovations improve the functionality, accuracy, and user-friendliness of pulse oximeters, making them increasingly attractive to both healthcare providers and patients.
Modern pulse oximeters incorporate sophisticated algorithms and signal processing technologies that significantly enhance the accuracy of oxygen saturation (SpO2) readings. Innovations such as Masimo's Signal Extraction Technology (SET) allow devices to deliver reliable measurements even under difficult conditions, such as low blood flow or patient movement. This level of reliability is vital in clinical settings where precise monitoring is essential for patient safety.
For instance, in December 2024, Masimo announced that its MightySat Medical has received FDA clearance, making it the first and only medical-grade fingertip pulse oximeter available over-the-counter (OTC) directly to consumers without a prescription. Unlike other pulse oximeters found in drug stores or online, which are not FDA-cleared and may provide inaccurate readings, the MightySat Medical is powered by Masimo SET pulse oximetry. This is the same advanced technology trusted by hospitals and clinics worldwide to monitor over 200 million patients each year, and has been proven to deliver accurate results without clinically significant differences across different skin tones.
Moreover, the rising demand for integration with telehealth solutions contributes to the global pulse oximeter market expansion.
Pulse Oximeter Market Dynamics: Restraints
Product recalls
The product recalls will hinder the growth of the global pulse oximeter market. Product recalls in the pulse oximeter market underscore significant safety concerns that can adversely affect consumer trust and overall market dynamics. A notable instance is the GE HealthCare recall of over 81,000 TruSignal pulse oximetry sensors, which highlights the potential dangers associated with faulty devices. This recall was classified as a Class I event by the FDA, indicating that these devices could pose serious risks to patients, including the possibility of worsening medical conditions or even death.
The recall was initiated after it was discovered that some TruSignal sensors might malfunction, particularly during critical situations such as defibrillation. Such malfunctions could hinder the defibrillator's ability to deliver necessary energy to a patient’s heart, potentially obstructing essential therapy during life-threatening emergencies.
Similarly, in April 2024, the Global Fund Quality Assurance and Compliance Team received urgent information regarding a recall initiated by Masimo Corporation for certain Rad-G Pulse Oximeter devices. The recall was prompted by an investigation that identified a significant issue some of these devices could unintentionally change their power state, meaning they might turn off or on without the user pressing the power button.
The problem with the Rad-G pulse oximeters involves them powering off unexpectedly, which can lead to a loss of monitoring capabilities. This situation poses serious risks, especially in clinical settings where continuous monitoring of patients is critical for timely medical intervention. Thus, the above factors could be limiting the global pulse oximeter market's potential growth.
Pulse Oximeter Market Segment Analysis
The global pulse oximeter market is segmented based on product type, technology, component, age group, end-user, and region.
Product Type:
The portable pulse oximeter product type segment is expected to hold 54.4% of the global pulse oximeter market in 2024
This segment encompasses devices that are compact, user-friendly, and suitable for continuous monitoring of oxygen saturation levels across various environments, including hospitals, home care, and long-term care facilities. It is fueled by several key factors that enhance its appeal to both healthcare providers and consumers.
Portable pulse oximeters are designed for ease of use and portability, making them ideal for patients who need to monitor their oxygen levels outside clinical settings. Their compact design facilitates easy transport, allowing patients to track their health at home or while traveling. There are 3 types of portable pulse oximeters fingertip pulse oximeter, a handheld pulse oximeter, and a wearable pulse oximeter.
The growing trend towards home healthcare has significantly boosted the demand for portable pulse oximeters. As more individuals manage chronic conditions such as Chronic Obstructive Pulmonary Disease (COPD) and asthma at home, the need for reliable monitoring devices has become crucial. Portable pulse oximeters offer a practical solution for self-monitoring oxygen saturation levels.
For instance, in August 2024, Prevounce Health launched its first blood oxygen device for remote patient monitoring (RPM), named the Pylo OX1-LTE. This new pulse oximeter is designed to enhance the management of patients with chronic respiratory conditions, such as chronic obstructive pulmonary disease (COPD) and asthma, as well as other health scenarios that require continuous monitoring and treatments that necessitate heart rate tracking. These factors have solidified the segment's position in the global pulse oximeter market.
Pulse Oximeter Market Geographical Analysis
North America is expected to hold 45.3% of the global pulse oximeter market in 2024
The region has a high incidence of chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD) and asthma. This prevalence drives the demand for effective monitoring solutions like pulse oximeters, which are essential for managing these conditions.
In this region, a major number of key players' presence, well-advanced healthcare infrastructure, government initiatives & regulatory support, product launches & approvals, and collaborations & partnerships would propel the pulse oximeter market growth. For instance, in March 2024, Movano Health completed a hypoxia trial in collaboration with the University of California, San Francisco (UCSF), which yielded promising results regarding the accuracy of its Evie Ring pulse oximeter. This trial confirmed that the Evie Ring's blood oxygen saturation (SpO2) measurements exceed the accuracy guidelines set by the U.S. Food and Drug Administration (FDA).
Similarly, in May 2024, Masimo announced the FDA clearance for its new baby monitoring system called Stork, designed specifically for healthy infants aged 0 to 18 months. This innovative device allows parents and caregivers to monitor key vital signs without the need for a prescription, making it accessible for home use. Thus, the above factors are consolidating the region's position as a dominant force in the global pulse oximeter market.
Asia-Pacific is expected to hold 18.9% of the global pulse oximeter market in 2024
Asia Pacific holds the fastest pace in the global pulse oximeter market and is expected to hold the most of market share. The rise in respiratory conditions, particularly chronic obstructive pulmonary disease (COPD) and asthma, significantly boosts the demand for pulse oximeters.
These devices are crucial for monitoring blood oxygen levels and essential for managing respiratory health effectively. The World Health Organization has reported that COPD is a leading cause of death globally, with a substantial burden falling on low- and middle-income countries where the need for monitoring tools is critical.
Countries like China and India are experiencing rapid population growth alongside an aging demographic that is more vulnerable to chronic diseases. This demographic shift increases the demand for medical devices such as pulse oximeters, which facilitate ongoing health monitoring in both hospital and home care settings.
Furthermore, key players in the industry product launching products that would drive this global pulse oximeter market growth. For instance, in February 2023, Heal Force introduced the FS-E2 Pulse Oximeter, a new product designed to effectively meet the blood oxygen saturation detection needs of residents, particularly elderly patients.
This device is capable of real-time monitoring of blood oxygen saturation and pulse rate, allowing for the timely detection of silent hypoxic symptoms in older adults in China. Thus, the above factors are consolidating the region's position as the fastest-growing force in the global pulse oximeter market.
Pulse Oximeter Market Major Players
The major global players in the pulse oximeter market include Masimo, Medtronic plc, Koninklijke Philips N.V., Nonin, NIHON KOHDEN CORPORATION., GE Healthcare, CONTEC MEDICAL SYSTEMS CO., LTD, ChoiceMMed, Drägerwerk AG & Co. KGaA, and ICU Medical, Inc., among others.
Key Developments
In December 2024, Zynex completed its clinical verification trial for the NiCO laser-based pulse oximeter, marking a major step toward bringing this innovative device to market. The NiCO pulse oximeter is designed to measure blood oxygen levels more accurately than traditional devices by using advanced laser technology rather than conventional LEDs.
In December 2024, Nonin Medical received FDA clearance for its TruO2 OTC fingertip pulse oximeter, making it the first over-the-counter (OTC) device of its kind designed and validated to provide accurate blood oxygen readings for patients of all skin colors. This clearance is significant because it addresses a longstanding issue: many traditional pulse oximeters have been shown to provide less accurate readings for individuals with darker skin, which can lead to delayed or inappropriate medical care.
In December 2024, Movano Health received FDA 510(k) clearance for the pulse oximeter function in its EvieMED Ring. This is a significant milestone for both the company and the wearable health device industry.
In May 2024, Masimo announced a partnership with Medable Inc. to integrate its medical-grade wearable devices into clinical research. This collaboration involves the incorporation of Masimo’s MightySat Rx pulse oximeter into Medable's evidence-generation platform for eight large-scale pharmaceutical clinical trials focused on two oncology indications: breast cancer and lung cancer.
Market Scope
Metrics | Details | |
CAGR | 7.9% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Product Type | Portable Pulse Oximeter, Tabletop/Bedside Pulse Oximeter |
Technology | Conventional Devices, Connected Devices | |
Component | Monitors, Sensors | |
Age Group | Adults, Pediatrics | |
End-User | Hospitals, Homecare Settings, Ambulatory Surgical Centers, Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and Middle East & Africa | |
The global pulse oximeter market report delivers a detailed analysis with 60+ key tables, more than 50 visually impactful figures, and 176 pages of expert insights, providing a complete view of the market landscape.