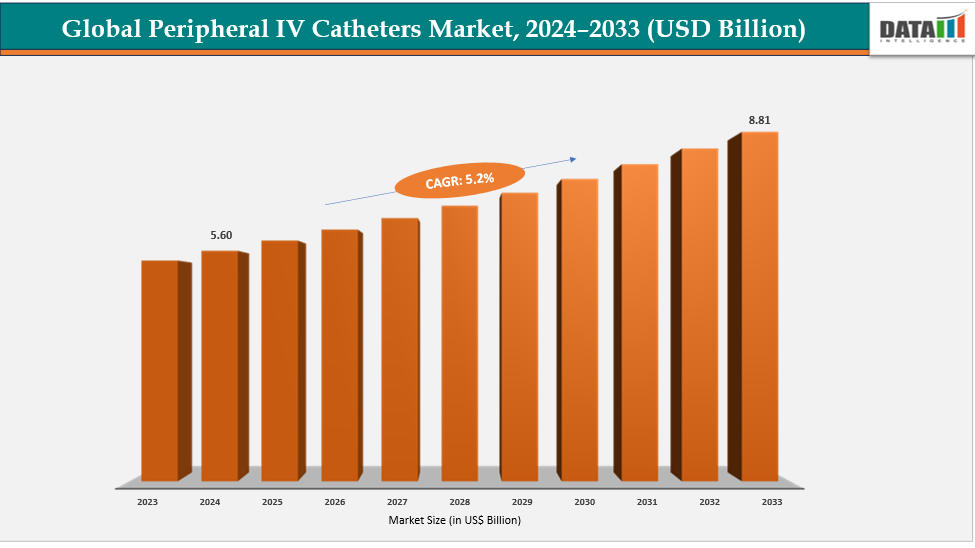
Peripheral IV Catheters Market Size & Industry Outlook
The growing number of surgical operations globally is driving the expansion of the peripheral IV catheters market, as intravenous access is required for anaesthesia, fluid administration, and medicine delivery during surgery. To properly manage larger patient numbers, hospitals and outpatient surgery centers require dependable, safety-engineered catheters. Minimally invasive and sophisticated operations boost the demand for PIVCs that are longer-lasting, infection-resistant, and safer. Furthermore, the increased number of elective operations, elderly populations requiring numerous treatments, and advancements in perioperative care are encouraging healthcare professionals to store more catheters.
Key Highlights
- North America is dominating the Global Peripheral IV Catheters Market with the largest revenue share of 48.5% in 2024
- The Asia Pacific region is the fastest-growing region in the Global Peripheral IV Catheters Market, with a CAGR of 7.7% in 2024
- The short peripheral IV catheters are dominating the peripheral IV catheters market with a 45.8% share in 2024
- The safety PIVCs segment is dominating the peripheral IV catheter market with a 58.3% share in 2024
- Top companies in the peripheral IV catheters market are Terumo Medical Corporation, Medline Industries, LP, B. Braun SE, Vygon SA, ICU Medical, Inc., Bound Tree Medical, LLC, NIPRO, ENVI Health Solutions, Polymed, and BD, among others.
Peripheral IV Catheters Market Executive Summary
Market Dynamics
Drivers: Technological advancements in catheter design are accelerating the growth of the peripheral IV catheter market
The peripheral IV catheter market is expanding at a rapid pace due to technological developments in catheter design. Safety-engineered catheters with retractable needles and protected designs prevent needlestick injuries, therefore enhancing healthcare worker safety and regulatory compliance. Closed-system catheters assist to reduce contamination and bloodstream infections, which improves patient safety and lowers hospital expenses. Flexible polyurethane and Teflon materials, for example, increase patient comfort and enable longer dwell periods. Integrated extension sets and color-coded hubs make it easier to insert and identify devices, improving clinical efficiency.
Owing to the factors like technological advancements and rising FDA approvals. For instance, in September 2024, B. Braun Medical Inc. received FDA 510(k) clearance for the Introcan Safety 2 Deep Access IV Catheter. The longer-length catheter, featuring fully automatic needlestick protection and multi-access blood control, was designed to increase dwell time and enhance patient safety.
Restraints: The risk of catheter-associated infections is hampering the growth of the peripheral IV catheter market
The growth of the global peripheral IV catheter market is restrained by the risk of catheter-associated infections (CAIs). Improper insertion, poor maintenance, or prolonged catheter use can lead to bloodstream infections, phlebitis, and thrombophlebitis, increasing patient morbidity and healthcare costs. Hospitals and clinicians are cautious in adopting PIVCs due to liability concerns and stringent infection-control protocols.
Consequently, safety concerns related to infections slow down market adoption, especially for innovative or expensive catheter systems, impacting overall market growth.
For more details on this report, see Request for Sample
Peripheral IV Catheters Market, Segmentation Analysis
The global peripheral IV catheters market is segmented based on product, technology, application, end‑user, and region
By Product: The short peripheral IV catheters are dominating the peripheral IV catheters market with a 45.8% share in 2024
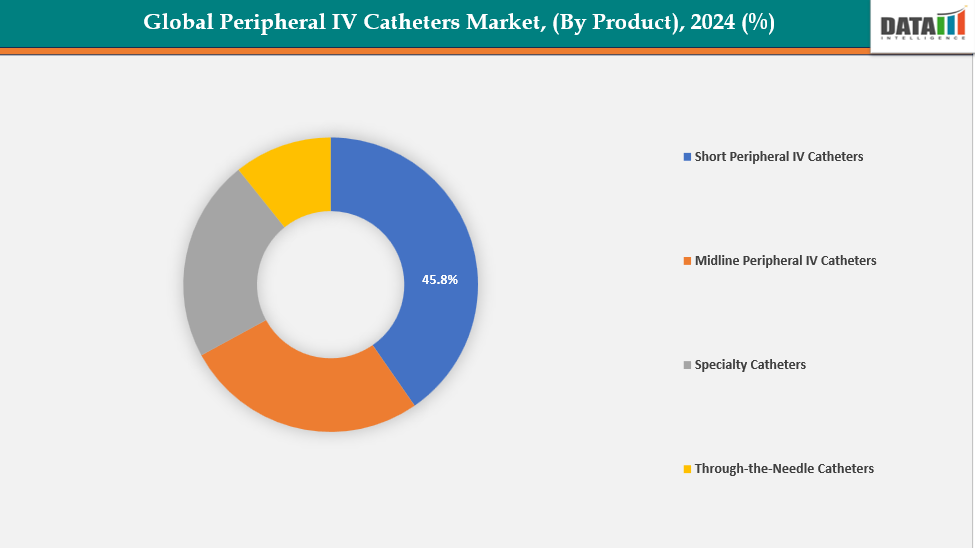
The worldwide market for peripheral IV catheters is dominated by short catheters due to they are inexpensive, simple to use, and appropriate for regular intravenous treatments. They are commonplace in hospitals, clinics, and outpatient settings because they are utilized for short-term fluid administration, drug delivery, and blood sample. Their rapid insertion and low training requirements enable effective patient turnover in high-volume healthcare facilities.
Additionally, short PIVCs are compatible with a wide range of infusion systems and safer for single-use protocols, reducing infection risk compared to more complex catheters. For instance, in November 2023, BD (Becton, Dickinson and Company) launched the PIVO Pro, a next-generation needle-free blood collection device. With FDA 510(k) clearance, it became the first to achieve compatibility with integrated and long peripheral IV catheters, advancing the company’s “One-Stick Hospital Stay” vision and enhancing patient care in U.S. hospitals.
By Technology: The safety PIVCs segment is dominating the peripheral IV catheter market with a 58.3% share in 2024
The safety PIVCs segment dominates the global peripheral IV catheter market due to increasing concerns over needlestick injuries and catheter-related infections among healthcare workers and patients. Safety-engineered designs, such as passive needle retraction, needle shields, and multi-access blood-control hubs, reduce exposure to bloodborne pathogens, enhancing workplace safety. Hospitals and clinics prefer these devices to comply with stringent regulatory standards and occupational safety guidelines.
Moreover, continuous technological innovation, new product launches, and rising FDA approvals make this segment dominant. For instance, in January 2024, B. Braun Canada launched the Introcan Safety 2 IV Catheter with Multi-Access Blood Control, featuring automatic needlestick protection and a blood control mechanism that minimized clinicians’ exposure to bloodborne pathogens during IV procedures.
Peripheral IV Catheters Market, Geographical Analysis
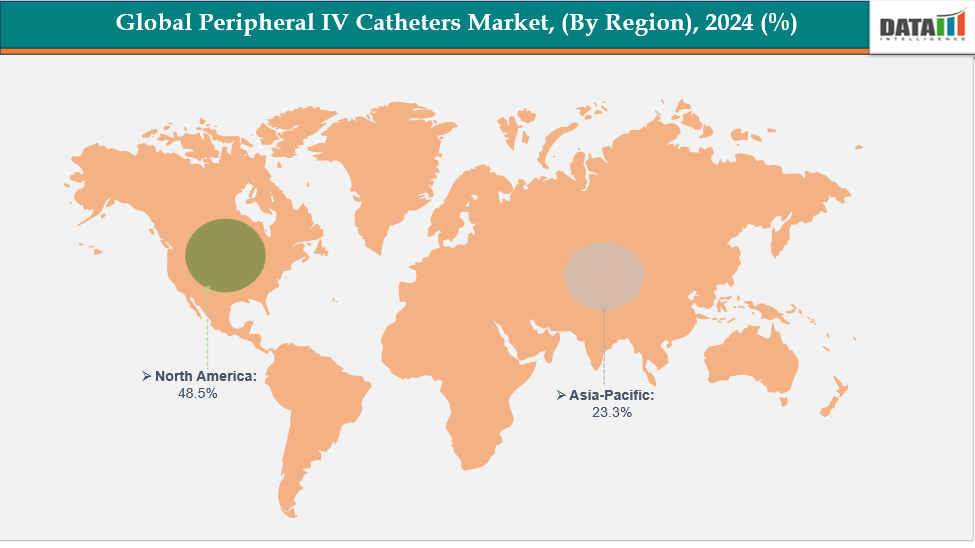
North America is dominating the global peripheral IV catheters market with 48.5% in 2024
North America leads the peripheral IV catheter market due to advanced healthcare infrastructure, high adoption of digital infusion and imaging technologies, and rising surgical and critical care cases. Favorable reimbursement policies, continuous product innovations, skilled medical personnel, and strict regulatory compliance further strengthen the region’s market dominance.
The U.S. peripheral IV catheters market is growing due to advanced healthcare infrastructure, frequent product launches, innovative technologies, and positive FDA 510(k) clearances, improving diagnostic, infusion, and critical care efficiency. For instance, in May 2025, SkyDance Vascular received FDA 510(k) clearance for its Osprey Midline Closed IV Catheter System, an extended dwell device building on previous Osprey models, advancing midline catheter technology, and enhancing patient safety in PIVC procedures.
Europe is the second region after North America, which is expected to dominate the Global Peripheral IV Catheters Market with 34.5% in 2024
The peripheral IV catheter market in Europe is growing due to advanced healthcare systems, a rising elderly population, and increased surgical procedures. Continuous product launches, favorable reimbursement policies, company mergers, acquisitions, and EU/CE approvals drive innovation, enhance patient safety, and support regional market expansion across hospitals and outpatient care settings.
Owing to factors like company mergers and acquisitions, for instance, in February 2025, Teleflex announced it had agreed to acquire substantially all of BIOTRONIK’s Vascular Intervention business for approximately €760 million, with the acquisition subject to regulatory approvals and expected to be complete by the third quarter of 2025.
The Asia Pacific region is the fastest-growing region in the Global Peripheral IV Catheters Market, with a CAGR of 7.7% in 2024
The Global Peripheral IV Catheters market in the Asia-Pacific region, including China, India, Japan, and South Korea, is growing rapidly due to increased healthcare spending, advanced hospital infrastructure, technological innovations, supportive government policies, and rising adoption of intravenous therapy and vascular access solutions in both urban and rural healthcare facilities.
In India, peripheral IV catheter companies are partnering with hospitals and clinics to improve vascular access, implement safety-engineered devices, enhance patient outcomes, and expand adoption in critical care, surgical, and outpatient settings.
Peripheral IV Catheters Market Competitive Landscape
Top companies in the peripheral IV catheters market are Terumo Medical Corporation, Medline Industries, LP, B. Braun SE, Vygon SA, ICU Medical, Inc., Bound Tree Medical, LLC, NIPRO, ENVI Health Solutions, Polymed, and BD, among others.
Terumo Medical Corporation: Terumo Medical Corporation is a global leader in medical devices, specializing in vascular access and infusion solutions. Its Peripheral IV Catheter portfolio includes safety-engineered and standard short-term catheters designed for reliable intravenous therapy, reducing needlestick injuries and improving patient outcomes. Terumo emphasizes innovation, quality, and compliance, supplying hospitals and clinics worldwide with advanced, safe, and efficient IV catheter solutions.
Key Developments:
- In November 2025, FUJIFILM Sonosite launched PIV Assist, an AI-powered feature designed to help clinicians plan peripheral IV access procedures. The technology was integrated into Sonosite LX, PX, and ST ultrasound systems, enhancing procedure efficiency, accuracy, and patient safety in peripheral intravenous catheter placement.
- In November 2025, Arctx Medical received FDA 510(k) clearance for its Arctx Cool Catheter Set, enabling nonsurgical, nasogastrically placed internal temperature management. The device circulates cold or warm water in a closed circuit, providing patient cooling or warming without general anesthesia or vascular access.
Market Scope
| Metrics | Details | |
| CAGR | 5.2% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | By Product | Short Peripheral IV Catheters, Midline Peripheral IV Catheters, Specialty Catheters, Through‑the‑Needle Catheters |
| By Technology | Safety PIVCs, Conventional PIVCs | |
| By Application | Intravenous Therapy, Blood Transfusion, Drug Administration, Critical Care, Others | |
| By End‑User | Hospitals, Ambulatory Surgical Centers, Home Healthcare, Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global peripheral IV catheters market report delivers a detailed analysis with 70 key tables, more than 67 visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more medical device-related reports, please click here