Molluscum Contagiosum Treatment Market Size
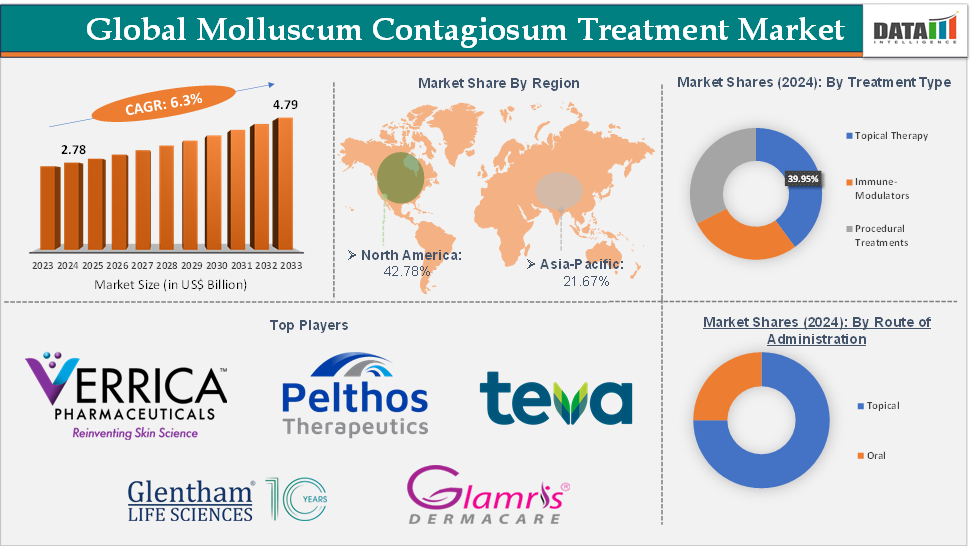
Molluscum Contagiosum Treatment Market size reached US$ 2.78 Billion in 2024 and is expected to reach US$ 4.79 Billion by 2033, growing at a CAGR of 6.3% during the forecast period 2025-2033.
Molluscum Contagiosum Treatment Market Overview
The global molluscum contagiosum treatment market is expected to grow owing to the continued approvals of first-in-class agents (e.g., nitric-oxide donors), expanded labeling for older products into pediatric cohorts, and growing patient/physician awareness will underpin growth. Adoption in emerging regions such as Asia-Pacific, Latin America, and the expansion of homecare distribution channels will further bolster market expansion.
The molluscum contagiosum treatment landscape is rapidly evolving, transitioning from predominantly physical modalities to targeted, non-invasive topical therapies backed by robust clinical data. With multiple novel agents approved since 2023 and several in late-stage pipelines, the market is poised for sustained growth underpinned by patient demand for safe, effective, and convenient treatments.
Executive Summary
For more details on this report – Request for Sample
Molluscum Contagiosum Treatment Market Dynamics: Drivers & Restraints
The rising preference for non-invasive treatments is significantly driving the molluscum contagiosum treatment market growth
Patients and caregivers increasingly favor topical creams and gels over procedures like curettage or cryotherapy because they’re painless, can be applied at home, and reduce the risk of scarring, especially important in young children, who make up over 80% of molluscum cases.
For instance, YCANTHE (a standardized cantharidin solution approved by the FDA in March 2023) comes with a single-use applicator and allows parents to treat lesions themselves without multiple clinic visits or the discomfort of physical removal. Similarly, ZELSUVMI (berdazimer sodium gel), approved in January 2024 as the first nitric-oxide–releasing topical for molluscum contagiosum, offers once-daily application with minimal irritation, avoiding the pain and anxiety of in-office procedures.
Non-invasive treatments offer self-administration, reducing the need for dermatology clinic visits. This is especially relevant in areas with limited access to specialists or during situations like the COVID-19 pandemic that encouraged remote care. OTC options like salicylic acid, benzoyl peroxide, and now prescription options like ZELSUVMI provide practical, home-based solutions for lesion management.
Overall, the growing preference for non-invasive treatments is accelerating market growth by fulfilling a critical need for safe, pain-free, and user-friendly therapies. The approvals of YCANTH and ZELSUVMI demonstrate how innovation in topical therapies is reshaping the molluscum contagiosum treatment landscape, shifting from clinic-based procedures to patient-centered, home-use solutions, thereby significantly expanding the market.
Availability of limited approved treatment options is hampering the molluscum contagiosum treatment market
Until 2023, there were no FDA-approved cantharidin formulations. Dermatologists typically used compounded cantharidin solutions (variable strength, purity, and packaging) or performed curettage/cryotherapy in the clinic. This not only introduced inconsistency in dosing but also discouraged some parents, especially of young children, from seeking treatment due to the pain and need for repeated office visits.
Commonly prescribed creams like imiquimod, podophyllotoxin, or retinoids have never been officially approved for molluscum contagiosum. Because their safety and efficacy were established primarily in small, uncontrolled studies, many dermatologists hesitate to use them as first-line options. This uncertainty leads to delays in treatment or a “watch-and-wait” approach, perpetuating the belief that molluscum contagiosum must simply run its natural course.
Even after YCANTH (VP-102) (cantharidin 0.7%) was approved in March 2023, its labeling initially covered children age ≥ 2 years, leaving infants and toddlers without a formally sanctioned option. Likewise, berdazimer sodium gel (ZELSUVMI) approval in January 2024 focused on patients aged ≥ 6 years. Because over 80% of MC cases occur in children under 8, the absence of approved treatments for the youngest cohorts forces providers to improvise or delay therapy, undermining market uptake.
As a result, the paucity of rigorously tested, approved treatments has constrained both clinician confidence and patient demand. Only when newer agents (e.g., YCANTH, ZELSUVMI) receive broader labeling and standardized reimbursement will the market overcome these roadblocks and scale more rapidly.
Molluscum Contagiosum Treatment Market Segment Analysis
The global molluscum contagiosum treatment market is segmented based on type, treatment type, route of administration, and region.
The topical therapy from the treatment type segment is expected to hold 39.95% of the market share in 2024 in the molluscum contagiosum treatment market
Over 80% of molluscum cases occur in children under 8 years, making painful in-office procedures (like cryotherapy or curettage) less desirable. Topical treatments offer a painless, at-home solution, which accelerates the segment growth. For instance, YCANTH (cantharidin 0.7%), FDA-approved in 2023, is applied with a precise single-use applicator and delivers over 80% lesion clearance within 12 weeks.
Advances in dermatology have led to the development of novel topical agents that are more effective and have fewer side effects. For instance, in January 2024, Ligand Pharmaceuticals Incorporated cleared the U.S. Food and Drug Administration (FDA) approval for ZELSUVMI (berdazimer topical gel, 10.3%) for the treatment of molluscum contagiosum (molluscum) in adults and pediatric patients one year of age and older. The FDA approved ZELSUVMI as the first novel drug for the treatment of molluscum infections. These FDA-backed topicals have boosted clinician confidence and accelerated adoption.
Topical therapies provide a painless alternative to invasive treatments like curettage and cryotherapy. This is particularly important for children, who make up a large percentage of molluscum contagiosum cases, as well as for individuals who prefer treatment options that do not involve discomfort or scarring. Parents and healthcare providers often favor topical options because they minimize pain and reduce the risk of complications, which is appealing for pediatric use.
Molluscum Contagiosum Treatment Market Geographical Analysis
North America is expected to dominate the global molluscum contagiosum treatment market with a 42.78% share in 2024
The U.S. was the first to see FDA approvals of key therapies such as YCANTH (cantharidin 0.7%) in March 2023 and ZELSUVMI (berdazimer sodium gel) in January 2024. Because these products hit U.S. pharmacies first, dermatologists and pediatricians began shifting away from patched-together, off-label regimens toward standardized, evidence-based topicals. For instance, within months of YCANTH’s launch, leading children’s hospitals in Texas and California started offering in-clinic treatment programs, something that didn’t exist before a formal approval made reimbursement possible.
Molluscum contagiosum is relatively common in North America, especially among children. The U.S. has one of the highest rates of diagnosis due to widespread access to healthcare and specialized dermatology services. For instance, according to the Pediatric Dermatology Research Alliance (PeDRA), molluscum contagiosum is most common in children. 6 million US children aged 0-14 years (5%-11.5% of children in this age group are affected by molluscum contagiosum. Molluscum affects up to 5% of the general population. North America’s proactive healthcare system ensures higher rates of diagnosis and treatment uptake, further driving market dominance in the region.
North America, particularly the U.S., is a hub for pharmaceutical research and innovation. Leading pharmaceutical companies and research institutions focus on developing targeted treatments for dermatological conditions, including molluscum contagiosum. For instance, Novan’s berdazimer gel (SB206), a promising nitric oxide-based topical gel under FDA review, reflects North American leadership in developing advanced treatments specifically for molluscum contagiosum. Such innovations could set a new standard for safe, effective therapies if approved, reinforcing the region’s dominance.
Asia-Pacific is growing at the fastest pace in the molluscum contagiosum treatment market, holding 21.67% of the market share
As in Western countries, parents in Asia-Pacific increasingly prefer safe, topical treatments over painful procedures like curettage or cryotherapy, especially for young children. This has created a strong market pull for products such as salicylic acid, retinoids, and newer agents under evaluation. Once approved in the region, FDA-cleared treatments like VP-102 (cantharidin 0.7%) and ZELSUVMI (berdazimer gel) are expected to see rapid uptake.
Regulatory bodies like Japan’s PMDA and South Korea’s MFDS are increasingly aligned with global standards, enabling faster approval of dermatological therapies. Japan is expected to be one of the first Asia-Pacific countries to approve Western-developed molluscum contagiosum treatments, setting the stage for broader regional access.
Molluscum Contagiosum Treatment Market Top Companies
Top companies in the molluscum contagiosum treatment market include Verrica Pharmaceuticals Inc., Pelthos Therapeutics, Teva Pharmaceutical Industries Limited, Glentham Life Sciences Limited, and Glamris Dermacare, among others.
Key Developments
In January 2024, Ligand Pharmaceuticals Incorporated cleared the U.S. Food and Drug Administration (FDA) approval for ZELSUVMI (berdazimer topical gel, 10.3%) for the treatment of molluscum contagiosum (molluscum) in adults and pediatric patients one year of age and older. The FDA approved ZELSUVMI as the first novel drug for the treatment of molluscum infections.
In July 2023, the U.S. Food and Drug Administration approved Verrica Pharmaceuticals Inc, to open a new tab treatment of a viral skin infection in adults and children aged 2 years and above. The green light for Verrica's drug, Ycanth, makes it the first approved treatment for the viral skin disease molluscum contagiosum in the United States. The FDA decision is a boost in the arm for the company after it previously failed to secure marketing approval for the drug, which is delivered through a single-use applicator, allowing for precise topical dosing and targeted administration.
Market Scope
Metrics | Details | |
CAGR | 6.3% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Type | MCV-1, MCV-2, MCV-3 and MCV-4 |
Treatment Type | Topical Therapy, Immune-Modulators, and Procedural Treatments | |
Route of Administration | Topical and Oral | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global molluscum contagiosum treatment market report delivers a detailed analysis with 55+ key tables, more than 55+ visually impactful figures, and 178 pages of expert insights, providing a complete view of the market landscape.