Global Lysosomal Storage Disorders (LSDs) Market: Industry Outlook
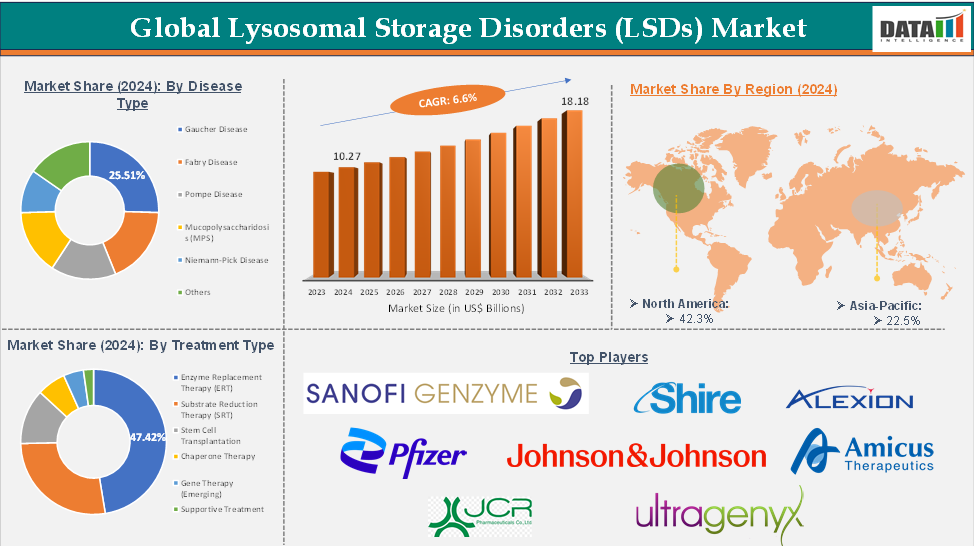
The global lysosomal storage disorders (LSDs) market reached US$ 9.68 Billion in 2023, with a rise of US$ 10.27 Billion in 2024 and is expected to reach US$ 18.18 Billion by 2033, growing at a CAGR of 6.6% during the forecast period 2025-2033.
The global lysosomal storage disorders (LSDs) market is expected to experience significant growth in the coming years due to a combination of clinical, technological, and commercial advancements. The market, which includes over 50 rare metabolic diseases, has gained attention from the pharmaceutical and biotechnology industries due to the unmet medical need and the potential for high-value treatments. The rising global prevalence of these diseases has led to earlier diagnosis and improved patient identification, supported by newborn screening programs and advances in genetic testing technologies.
The market has evolved from traditional enzyme replacement therapies to more advanced modalities such as substrate reduction therapy, pharmacological chaperones, gene therapy, and genome editing approaches. Key players like Sanofi Genzyme, Takeda, BioMarin, Amicus Therapeutics, and Alexion Pharmaceuticals have established a strong portfolio of commercial products, while a new wave of biotech innovators is expanding the pipeline through strategic collaborations, licensing deals, and cutting-edge R&D. However, the LSDs market still faces barriers such as high treatment costs, the rarity and complexity of these diseases, and the need for specialized infrastructure and multidisciplinary care.
Global Lysosomal Storage Disorders (LSDs) Market: Executive Summary
Global Lysosomal Storage Disorders (LSDs) Market Dynamics: Drivers, Restraints & Opportunities
Driver: Increasing prevalence of rare genetic disorders
The global lysosomal storage disorders (LSDs) market is experiencing growth due to the rise in rare genetic disorders. As awareness and diagnostic capabilities improve, more patients are being diagnosed with conditions previously under-recognized or misdiagnosed, such as Gaucher disease, Fabry disease, Pompe disease, and mucopolysaccharidoses (MPS). This expansion in patients eligible for targeted therapies increases the demand for effective treatment options.
Advancements in genetic testing, such as next-generation sequencing, have enabled earlier identification of LSDs, facilitating timely intervention. Governments and healthcare systems are also prioritizing rare disease management, funding research, improving patient registries, and supporting orphan drug development. This has led to pharmaceutical companies investing in LSD therapies, highlighting the significant unmet medical need.
For instance, rare genetic disorders, affecting 3.5% to 5.9% of the global population, are among the approximately 7,000 known conditions, posing a significant burden on individuals, families, and healthcare systems, despite their individual rarity.
Driver: Advancements in enzyme replacement and gene therapies
The global lysosomal storage disorders (LSDs) market is growing due to advancements in enzyme replacement therapy (ERT) and gene therapies. ERT has been the primary treatment for LSDs like Gaucher, Fabry, and Pompe, improving patient quality of life. Improvements in enzyme formulation, delivery methods, and dosing regimens have improved their efficacy, safety, and convenience. Gene therapy, on the other hand, offers potential for long-term or even curative treatment through single-administration interventions.
Companies are developing adeno-associated virus (AAV) and lentiviral-based gene therapies to correct genetic defects responsible for enzyme deficiencies. These therapies are advancing rapidly through clinical trials and receiving regulatory support. This shift in the therapeutic landscape is expanding treatment options and attracting significant investment and partnerships in the biopharmaceutical sector.
Restraint: High treatment and therapy costs
The global lysosomal storage disorders (LSDs) market faces significant challenges due to the high cost of treatments and therapies, particularly enzyme replacement therapies and gene therapies. These costs, often ranging from hundreds of thousands to millions of dollars per patient, burden healthcare systems, insurers, and patients, particularly in low- and middle-income countries.
Limited reimbursement policies, lack of universal healthcare access, and disparities in healthcare infrastructure further exacerbate the affordability of these life-saving treatments, hindering early diagnosis and limiting the market's growth potential.
Opportunity: Growth in gene therapy and personalized medicine
Emerging markets, including Asia-Pacific, Latin America, the Middle East, and parts of Africa, are experiencing rapid economic development, rising healthcare awareness, and increased investment in The Global Lysosomal Storage Disorders (LSDs) Market is being significantly transformed by gene therapy and personalized medicine. Traditional treatments, like enzyme replacement therapy, focus on managing symptoms and slowing disease progression but do not address the root genetic causes of LSDs.
Moreover, advances in genomics, bioinformatics, and molecular diagnostics are accelerating the identification of specific mutations and patient stratification strategies, making it easier to design customized therapeutic plans. As gene therapy products gain regulatory approvals and personalized approaches become more mainstream, they are expected to drive a paradigm shift in LSD treatment, from symptom management to disease modification and potential cures.
For more details on this report, Request for Sample
Global Lysosomal Storage Disorders (LSDs) Market Segment Analysis
The global lysosomal storage disorders (LSDs) market is segmented based on disease type, treatment type, route of administration, end user, and region.
Treatment Type:
The enzyme replacement therapy (ERT) segment is expected to hold 34.25% share of the lysosomal storage disorders (LSDs) market
The enzyme replacement therapy (ERT) segment is a significant player in the global lysosomal storage disorders (LSDs) Market due to its clinical efficacy and role in managing various LSDs like Gaucher disease, Fabry disease, and Pompe disease. ERT involves intravenous administration of recombinant enzymes to compensate for deficient or absent enzymes, alleviating disease symptoms and slowing progression.
Its long-standing clinical use and favorable reimbursement policies in developed regions have led to strong adoption rates. The increasing diagnosis rates, newborn screening programs, and patient awareness further support the demand for ERT. Advancements in ERT formulations enhance treatment outcomes and patient compliance.
Global Lysosomal Storage Disorders (LSDs) Market - Geographical Analysis
North America dominated the global lysosomal storage disorders market with the highest share of 42.3% in 2024
North America, particularly the United States, holds a significant market position in the Global Lysosomal Storage Disorders (LSDs) market due to advanced healthcare infrastructure, high disease awareness, and strong research and development capabilities. The region is home to leading biopharmaceutical companies, academic research institutions, and regulatory frameworks like the U.S. FDA, which encourage rare disease therapies. Specialized treatment centers and genetic counseling services facilitate early diagnosis and intervention.
Moreover, North America's robust insurance and reimbursement policies cover high-cost treatments like enzyme replacement therapy and emerging gene therapies, increasing patient access. Strong investment in gene therapy and personalized medicine startups, strategic partnerships, and patient advocacy groups contributes to therapeutic innovation.
Asia-Pacific is the global lysosomal storage disorders (LSDs) market with a market share of 22.5% in 2024
The Asia Pacific region is experiencing a rapid growth in the market for Lysosomal Storage Disorders (LSDs), driven by improved healthcare infrastructure, rising healthcare expenditure, and increased awareness of rare genetic disorders. Countries like China, India, Japan, and South Korea are implementing early disease detection initiatives, including newborn screening programs and genetic diagnostics advancements.
For instance, in June 2025, JCR Pharmaceuticals Co., Ltd., a global biopharmaceutical company, launched a new global website to celebrate its 50th anniversary. The website, designed for global audiences, showcases the company's commitment to rare and genetic diseases, highlighting its presence in Japan, the U.S., Europe, and Latin America, and bringing the JCR brand to life for patients, physicians, and partners worldwide.
Despite underreporting due to limited diagnostic capabilities, improvements in healthcare access and education are improving identification and reporting of LSDs. Government-backed programs and policy reforms are creating a favorable regulatory environment. Asia Pacific offers cost advantages in clinical research and manufacturing, attracting multinational pharmaceutical and biotech companies to expand operations and clinical trials. Collaborations between global and local companies and the rise of domestic biotech firms focused on rare diseases are accelerating treatment development.
Global Lysosomal Storage Disorders (LSDs) Market - Key Players
The major global players in the lysosomal storage disorders (LSDs) market include Sanofi Genzyme, Shire, Alexion Pharmaceuticals, Inc., Johnson & Johnson, Pfizer Inc., Amicus Therapeutics, Inc., JCR Pharmaceuticals Co., Ltd, Ultragenyx Pharmaceutical Inc
Global Lysosomal Storage Disorders (LSDs) Market – Key Developments
In September 2024, Chiesi Global Rare Diseases, a division of the Chiesi Group, launched a new Research Grant Initiative, Find For Rare, allowing researchers to apply for up to €50,000 to fund their studies on lysosomal storage disorders (LSDs).
Global Lysosomal Storage Disorders (LSDs) Market: Scope
Metrics | Details | |
CAGR | 6.6% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Disease Type | Gaucher Disease, Fabry Disease, Pompe Disease, Mucopolysaccharidosis (MPS), Niemann-Pick Disease, Others |
Treatment Type | Enzyme Replacement Therapy (ERT), Substrate Reduction Therapy (SRT), Stem Cell Transplantation, Chaperone Therapy, Gene Therapy (Emerging), Supportive Treatment | |
Route of Administration | Intravenous, Oral, Others | |
End User | Hospitals, Specialty Clinics, Research Institutes, Home Healthcare | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
Suggestions for Related Report
For more pharmaceuticals-related reports, please click here