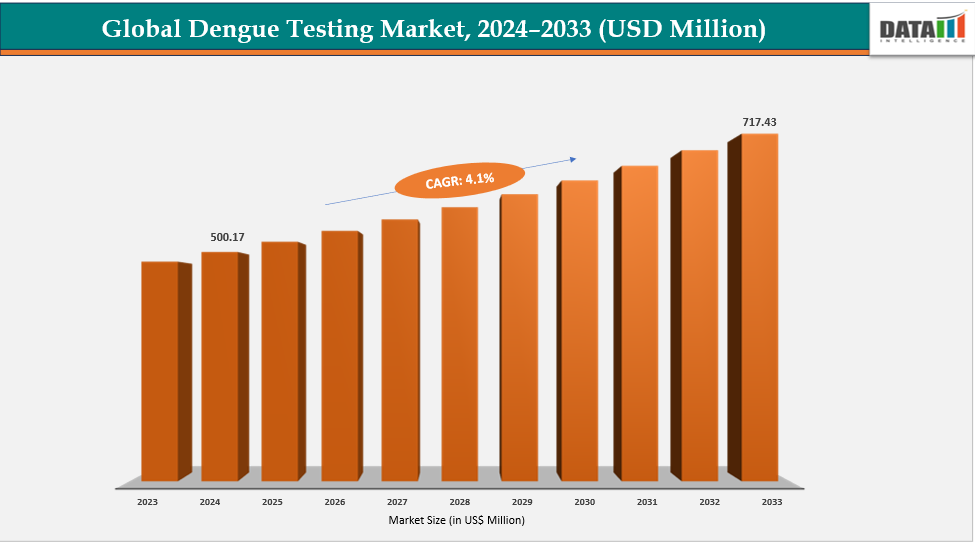
Dengue Testing Market Size & Industry Outlook
Advancements in diagnostic technologies are propelling the expansion of the dengue testing market. Rapid antigen and antibody testing kits utilizing lateral flow immunoassay are facilitating quicker screenings at point-of-care locations. Developments in multiplex detection are enabling the simultaneous identification of various serotypes. The incorporation of molecular platforms like RT-PCR and targeted sequencing is enhancing accuracy, genome monitoring, and tracking of outbreaks. Enhanced automation, AI-driven interpretation tools, cloud-based data analytics, and digital reporting systems are improving speed and decision-making processes. A rise in R&D investments focused on predictive biomarkers, markers for severe disease risk, and high-sensitivity NS1 detection is also fueling adoption.
Key Highlights
- North America is dominating the global dengue testing market with the largest revenue share of a 48.5% in 2024
- The Asia Pacific region is the fastest-growing region in the global dengue testing market, with a CAGR of 7.7% in 2024
- The ELISA segment is dominating the dengue testing market with a 42.3% share in 2024
- The rapid diagnostic tests segment is dominating the dengue testing market with a 55.3% share in 2024
- Top companies in the dengue testing market are F. Hoffmann-La Roche Ltd, Abbott, SD Biosensor, INC, Thermo Fisher Scientific Inc, CERTEST BIOTEC, EUROIMMUN Medizinische Labordiagnostika AG, InBios International, Inc, Stergic, Shanghai ZJ Bio‑Tech Co., Ltd., and AffiPCR Biosystems, among others.
Market Dynamics
Drivers: Rising prevalence and geographic spread of dengue are accelerating the growth of the dengue testing market
The growing occurrence and broader geographical distribution of dengue are significant drivers of expansion in the worldwide dengue testing market. Accelerated urban development, alterations in climate, and an increase in mosquito breeding have heightened outbreaks in tropical and subtropical areas, resulting in a critical need for prompt diagnosis. Authorities and public health organizations are prioritizing monitoring programs and early detection to manage epidemics, which is further increasing the adoption of testing. Innovations in technology, such as rapid diagnostic tests, molecular assays, and point-of-care solutions, are enhancing the speed, accuracy, and accessibility of testing in remote locations.
Owing to factors like prevalence, in 2024, WHO reported over 7.6 million dengue cases globally, including 3.4 million confirmed and more than 16,000 severe cases, with active transmission identified in 90 countries.
Restraints: Limited availability of effective diagnostic tools in lower economic regions is hampering the growth of the dengue testing market
At present, the effectiveness of dengue diagnostic test kits is hindered by the limited availability of dependable tools and the high costs associated with current tests. Traditional methods, including cell culture and inoculation in mice, tend to be slow, labor-intensive, and costly. This situation has led companies to concentrate on creating affordable and precise diagnostic options.
Additionally, the global market for dengue testing faces challenges, especially in low-income and resource-poor areas, where laboratories lack sufficient equipment, trained personnel are in short supply, and supply chains are unreliable. In rural and isolated regions, the absence of point-of-care facilities results in delays in diagnosis and treatment, heightening the risk of severe cases and outbreaks.
For more details on this report, see Request for Sample
Dengue Testing Market, Segment Analysis
The global dengue testing market is segmented based on technology, test type, end user and region
By Technology: The ELISA segment is dominating the dengue testing market with a 42.3% share in 2024
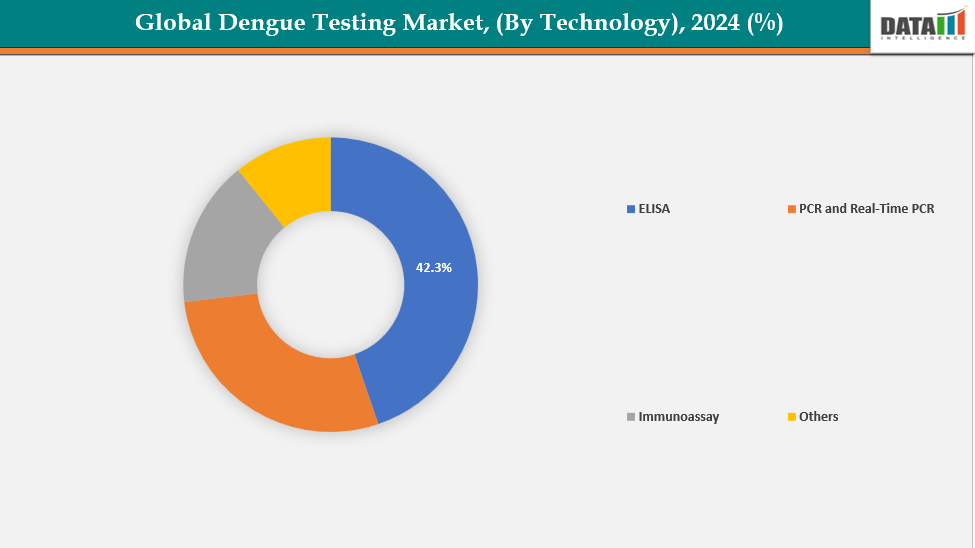
The ELISA (Enzyme-Linked Immunosorbent Assay) segment is leading the global dengue testing market due to its excellent sensitivity and specificity in identifying dengue antigens and antibodies. ELISA tests provide dependable results, facilitating early diagnosis and efficient patient management, which is essential in areas at risk of outbreaks. Their extensive use in hospitals, diagnostic laboratories, and public health initiatives is additionally fueling market expansion.
Additionally, Technological advancement in this segment, such as full test kits offered by several major players in the market, drives the market's growth. For example, PANBIO DENGUE IGG INDIRECT ELISA, a test kit offered by Abbott, is used to detect IgG antibodies to dengue antigen serotypes (1, 2, 3, and 4) in serum as an aid to the clinical laboratory diagnosis of patients with clinical symptoms and past exposure consistent with dengue fever. Another test kit offered by InBios International Inc. is the DENV Detect NS1 ELISA Kit, which is also used for the early detection of dengue virus (DENV) NS1 antigen in human serum.
By Test Type: The rapid diagnostic tests segment is dominating the dengue testing market with a 55.3% share in 2024
The segment of rapid diagnostic tests (RDTs) is at the forefront of the global dengue testing market due to their quick results, user-friendliness, and affordability. These tests allow healthcare professionals to identify dengue infections directly at the point of care, offering results in just a few minutes without requiring advanced laboratory equipment. Their straightforward application and low training necessities make them particularly suitable for deployment in remote and resource-poor areas, where the incidence of dengue is high.
Additionally, ongoing advancements in RDT technology and new product launches are strengthening their market position. For instance, in September 2025, CTK Biotech, part of SSI Diagnostica Group, received CE‑IVDR approval for its OnSite RSV Ag Rapid Test, marking a significant advancement in respiratory diagnostics and reinforcing its impact on global health.
Dengue Testing Market, Geographical Analysis
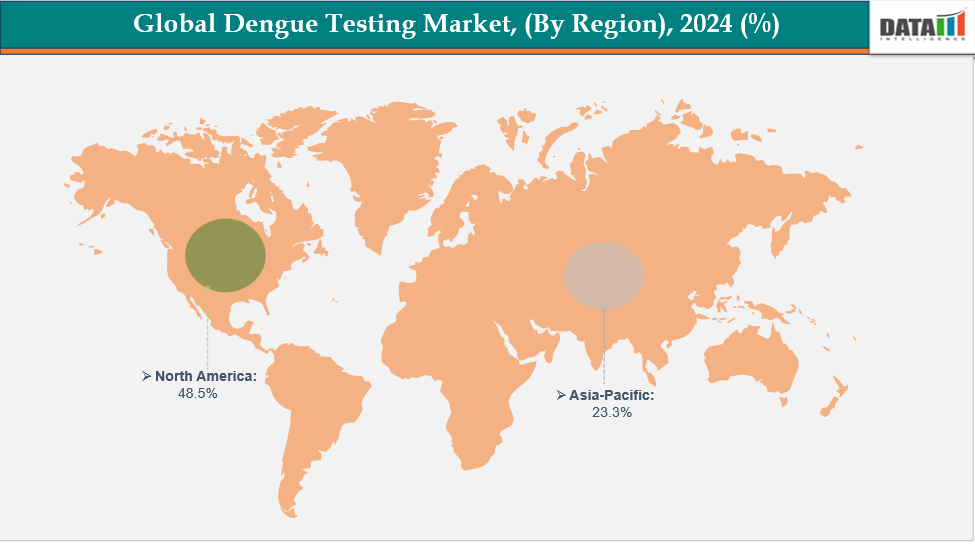
North America is dominating the global dengue testing market with a 48.5% in 2024
North America is at the forefront of the global dengue testing market due to of its well-developed healthcare infrastructure, the use of cutting-edge diagnostic technologies, and an increasing awareness of dengue. The region's market leadership is bolstered by supportive government programs, a workforce of skilled medical professionals, the regular launch of rapid diagnostic tests, and rigorous regulatory standards.
In the United States, the global dengue testing market is expanding due to the regular introduction of rapid diagnostic tests, the embrace of cutting-edge technologies, favorable FDA approvals, and government support for clinical trial funding. For instance, in February 2025, AbViro launched a federally funded Phase IIa dengue challenge trial (NCT05048875), enrolling 84 participants to evaluate its AV1 candidate, exposing volunteers to a weakened virus strain to advance dengue treatment research.
Europe is the second region after North America, which is expected to dominate the global dengue testing market with 34.5% in 2024
The market for dengue testing in Europe is growing as a result of improved healthcare facilities, heightened awareness about the disease, and more robust disease monitoring. The regular launch of rapid diagnostic tests, favorable reimbursement strategies, and rigorous EU and CE regulatory approvals are fostering innovation, enhancing diagnostic precision, and speeding up market expansion in the region.
Owing to factors like continuous EU and CE mark approvals, for instance, in October 2025, Roche received CE marking for its Elecsys Dengue Ag test, a high-throughput, fully automated immunoassay, advancing the efficient and reliable diagnosis of acute dengue infections and addressing the global dengue challenge.
The Asia Pacific region is the fastest-growing region in the global dengue testing market, with a CAGR of 7.7% in 2024
The dengue testing market in the Asia-Pacific region, which encompasses China, India, Japan, and South Korea, is growing swiftly as a result of the increasing cases of dengue, advancements in diagnostic technology, enhanced healthcare infrastructure, heightened awareness regarding early detection, and greater government efforts in disease monitoring and outbreak control.
In China, the market for dengue testing is growing, driven by the regular introduction of rapid diagnostic tests, the incorporation of cutting-edge technologies, approvals from the NMPA, government initiatives for managing outbreaks, and an increasing awareness of the importance of early dengue detection. Owing to factors like NMPA approvals, for instance, in October 2024, BioPerfectus’ Dengue Virus Real-Time PCR Kit received approval from China’s NMPA, enabling accurate qualitative detection of dengue virus nucleic acids in human serum and advancing reliable diagnostics for effective disease management.
Dengue Testing Market Competitive Landscape
Top companies in the dengue testing market are F. Hoffmann-La Roche Ltd, Abbott, SD Biosensor, INC, Thermo Fisher Scientific Inc, CERTEST BIOTEC, EUROIMMUN Medizinische Labordiagnostika AG, InBios International, Inc, Stergic, Shanghai ZJ Bio‑Tech Co., Ltd., and AffiPCR Biosystems, among others.
F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd is a global healthcare leader actively advancing the dengue testing market through innovative diagnostics. The company’s Elecsys Dengue Ag test, a high-throughput, fully automated immunoassay, aids in rapid and accurate detection of acute dengue infections. Roche focuses on enhancing laboratory efficiency, improving diagnostic reliability, and supporting early disease detection worldwide.
Key Developments:
- In January 2025, Visgene has been advancing research and development to bring antibody technology for identifying all four dengue virus serotypes into practical medical use. We are pleased to announce that our product, “VisCheck Dengue NS1 Rapid Antigen Serotyping Test,” has now received in vitro diagnostic device registration from the Food and Drug Administration of the Kingdom of Thailand. VisCheck Dengue NS1 Rapid Antigen Serotyping Test: World’s First Approval for Dengue Severe Disease Prediction Diagnostic Kit
- In April 2025, Boditech Med received ANVISA Approval for Four Diagnostic Kits for Dengue Fever and Latent TBBoditech Med, a leading provider of point-of-care diagnostics, announced on the 23rd that it has received product approval from Brazil’s National Health Surveillance Agency (ANVISA) for four diagnostic kits, including two for dengue fever and two for latent tuberculosis (LTBI).
- In November 2024, MGI Tech Co., Ltd. launched its ATOPlex DENV1-4 Targeted Sequencing Package, delivering a comprehensive solution for rapid and accurate dengue virus detection. The platform provided an end-to-end workflow from nucleic acid extraction to DNBSEQ sequencing and data analysis, enabling outbreak-supportive results in just 12 hours.
Market Scope
| Metrics | Details | |
| CAGR | 4.1% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | By Technology | ELISA, PCR and Real-Time PCR, Immunoassay, Others |
| By Test Type | Rapid Diagnostic Tests, Serological Tests, Molecular Tests, Others | |
| By End User | Hospitals and Clinics, Diagnostic Laboratories, Point-of-Care Testing Centers, Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global dengue testing market report delivers a detailed analysis with 62 key tables, more than 52 visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more clinical diagnostics-related reports, please click here