Cancer Gene Therapy Market Size& Industry Outlook
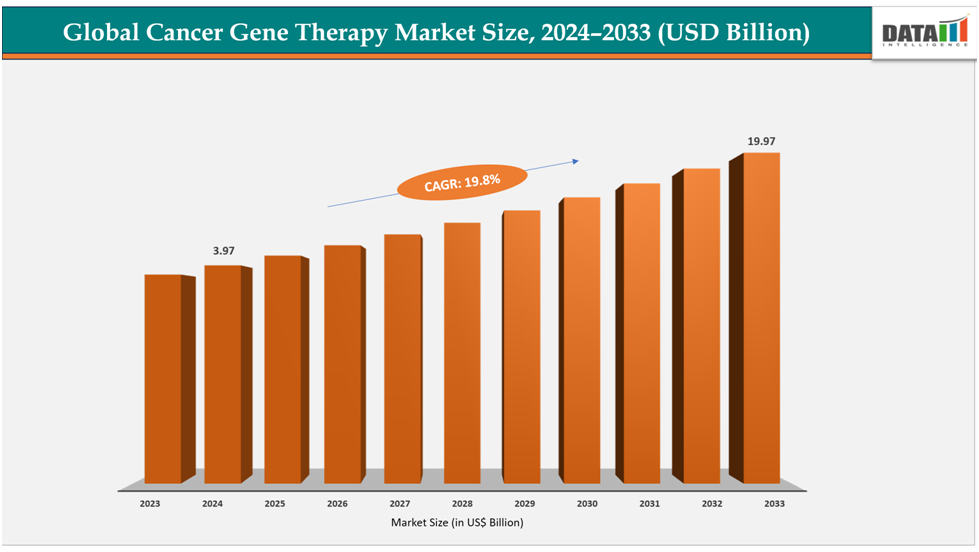
The global cancer gene therapy market size reached US$ 3.36 Billion with rise of US$3.97Billion in 2024 is expected to reach US$ 19.97Billion by 2033, growing at a CAGR of 19.8%during the forecast period 2025-2033.
Rapid technological advances in gene-editing and cell engineering are transforming the cancer gene therapy market by making treatments more precise, effective, and scalable. Breakthrough tools such as CRISPR/Cas9, TALENs, and zinc-finger nucleases enable highly accurate editing of cancer-related genes, minimizing off-target effects and improving safety.
At the same time, innovations in cell engineering such as next-generation CAR-T and TCR-T therapies are enhancing immune cells to better recognize and destroy tumors, resist immune suppression, and persist longer in the body. Furthermore, progress in viral and non-viral vector systems has improved gene delivery efficiency, while automation and advanced biomanufacturing platforms are streamlining production, reducing costs, and accelerating commercialization.
Key Highlights
North America dominates the cancer gene therapy market with the largest revenue share of 43.5% in 2024.
The Asia Pacific is the fastest-growing region and is expected to grow at the fastest CAGR of8.1% over the forecast period.
Based on therapy type, gene induced immunotherapy segmented the market with the largest revenue share of 44.1% in 2024.
The major market players in the Novartis AG, Pfizer, Adaptimmune Therapeutics, Bluebird Bio, Inc., Krystal Biotech, Inc, Regulus Therapeutics, Biogenera and among others.
Market Dynamics
Drivers: The rising investment, partnerships, and product approvals is significantly driving the cancer gene therapy market growth
The global cancer gene therapy market is being driven by increased investment, strategic partnerships, and product approvals. Major pharmaceutical companies, biotech firms, and venture capitalists are investing in research and development to accelerate gene-editing technologies and cell-based therapies. Collaborations between large pharma and specialized biotechs enable faster translation of laboratory breakthroughs into clinical Indications. Regulatory approvals of landmark therapies like Kymriah and Yescarta validate the clinical potential of gene therapies in oncology, encouraging more companies to pursue similar programs.
For instance, in May 2025, The US FDA approved nine cell and gene therapy products in 2024 to treat cancer, hemophilia B, and L-amino acid decarboxylase deficiency. This momentum continues into 2025 with Encelto for macular telangiectasia Therapy Type 2 and Zevaskyn for recessive dystrophic epidermolysis bullosa, providing hope to patients with limited treatment options.
Restraints: Regulatory complexity and safety/immune risks are hampering the growth of the cancer gene therapy market
Regulatory complexity and safety/immune risks remain significant challenges in the global cancer gene therapy market. Developing and approving these therapies involves navigating highly stringent and often inconsistent regulatory frameworks across different regions, which slows clinical progress and commercialization timelines. Safety concerns, such as cytokine release syndrome, neurotoxicity, and potential off-target genetic effects, also pose critical hurdles to patient adoption and physician confidence. Long-term monitoring requirements further complicate trial design and add to development costs. These challenges not only delay market entry but also limit accessibility, making it difficult for smaller biotech firms to compete with larger players.
For more details on this report – Request for Sample
Segmentation Analysis
The global cancer gene therapy market is segmented based on therapy type, indication, end user, and region.
Therapy Type:
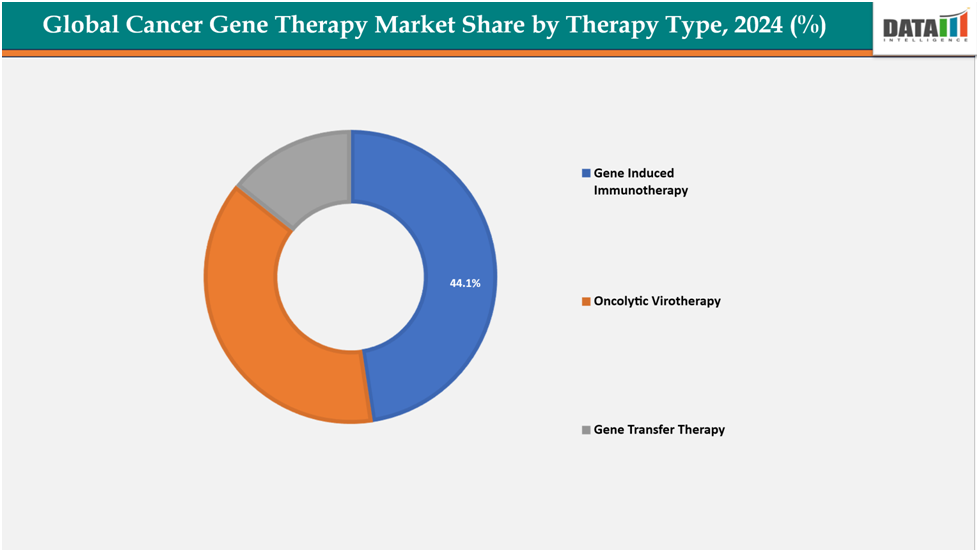
The gene induced immunotherapy from therapy type segment to dominate the cancer gene therapy market with a 44.1% share in 2024
The gene-induced immunotherapy segment is driving growth in the global cancer gene therapy market due to its proven ability to reprogram a patient’s own immune system to effectively recognize and attack tumors. CAR-T cell therapies like Novartis’ Kymriah and Gilead’s Yescarta have demonstrated high remission rates in relapsed or refractory blood cancers, where conventional treatments often fail.
These successes have validated the therapeutic approach, resulting in strong demand, rapid regulatory approvals, and accelerated R&D investment in expanding indications beyond hematological malignancies to solid tumors. The clinical efficacy and commercialization of these therapies are establishing gene-induced immunotherapy as the most dominant and revenue-generating segment within cancer gene therapy, making it a primary growth engine for the market.
Indication: The breast cancer segment is estimated to have a 31.4% of the cancer gene therapy market share in 2024
The breast cancer segment is driving growth in the global cancer gene therapy market due to the rising prevalence of the disease worldwide and the urgent need for more effective treatments beyond chemotherapy, radiotherapy, and hormonal therapies. Gene-based approaches, including oncolytic virotherapy and gene-modified immunotherapies, are being actively investigated to overcome resistance in aggressive forms such as triple-negative breast cancer (TNBC). Ongoing clinical trials using adenoviral vectors and gene-edited immune cells are showing encouraging results in improving tumor response and survival rates. Additionally, the strong focus of pharmaceutical and biotech companies on developing targeted gene therapies for breast cancer, coupled with increasing research funding and patient enrollment in clinical studies, is propelling this segment forward as one of the key contributors to overall market expansion.
Geographical Analysis
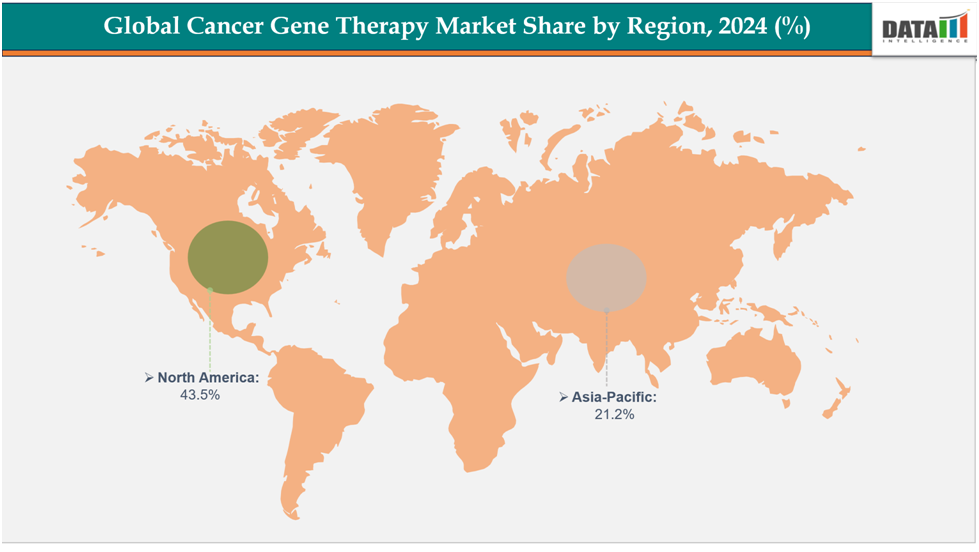
North America is expected to dominate the global cancer gene therapy market with a 43.5% in 2024
North America leads the global cancer gene therapy market, supported by high healthcare spending, strong clinical research infrastructure, and widespread adoption of advanced oncology treatments. The presence of major biotech firms and continuous funding for gene-editing technologies ensure steady market expansion.
For instance, in August 2024, The U.S. FDA has approved Tecelra, a gene therapy for adults with unrespectable or metastatic synovial sarcoma who have received prior chemotherapy, are HLA antigen positive, and express the MAGE-A4 antigen, as determined by FDA-approved diagnostic devices.
The U.S. dominates this space with FDA approvals of breakthrough therapies such as Kymriah and Yescarta. Extensive clinical trial activity, advanced manufacturing capabilities, and strong venture capital investment further strengthen its leadership in cancer gene therapy development and commercialization.
Europe is the second region after North America which is expected to dominate the global cancer gene therapy market with a 34.5% in 2024
The European innovation council (EIC) cell and gene therapy symposium is expected to significantly drive the growth of the European cancer treatment market. The event, organized in October 2023, brings together key stakeholders in the rapidly evolving cell and gene therapy sector to discuss advancements, challenges, and opportunities. It serves as a platform for researchers, clinicians, industry leaders, investors, and policymakers to exchange ideas, collaborate on innovative projects, and drive the development of cutting-edge therapies. The symposium is a crucial event in driving the growth of the European cancer treatment market.
Germany acts as a central hub for cancer gene therapy in Europe, thanks to its advanced healthcare system, strong research universities, and active participation in clinical trials. High patient awareness and access to innovative therapies contribute to its market strength.
Moreover, the growth of cancer treatment in the UK is driven by increased research investment, favorable regulatory frameworks, and rising cancer cases. Collaborations between academic institutions and biotech companies are accelerating the development of innovative therapies, with personalized medicine enhancing the potential of gene therapies for improved patient outcomes.
The Asia Pacific region is the fastest-growing region in the global cancer gene therapy market ,with a CAGR of 8.1% in 2024
Asia-Pacific is emerging as a high-growth region due to the rising cancer burden, improving healthcare infrastructure, and government initiatives to promote biotechnology innovation. Partnerships between global pharma companies and local players are accelerating therapy development and adoption.
Japan drives regional growth with its fast-track regulatory framework for regenerative and gene-based medicines. Early approvals of advanced therapies, combined with strong government support and academic-industry collaboration, make Japan a key market for cancer gene therapy in Asia.
For instance, the approval of Tecartus (brexucabtagene autoleucel) by the Japanese Ministry of Health, Labour and Welfare (MHLW) for relapsed or refractory mantle cell lymphoma, making Japan one of the earliest countries outside the U.S. to provide patient access to this CAR-T therapy. Such regulatory agility, coupled with strong government support and active collaboration between academia and industry, continues to position Japan as a leader in cancer gene therapy adoption.
Competitive Landscape
Top companies in the cancer gene therapy market include Novartis AG, Pfizer, Adapt immune Therapeutics, Bluebird Bio, Inc., Krystal Biotech, Inc., Regulus Therapeutics, Biogenera and among others.
Novartis AG :Novartis plays a pivotal role in the global cancer gene therapy market, primarily through its leadership in CAR-T cell therapies. The company’s flagship product, Kymriah (tisagenlecleucel), was the first FDA-approved CAR-T therapy for pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) and later expanded to treat adult patients with large B-cell lymphoma. Novartis has invested heavily in developing scalable manufacturing platforms, global distribution networks, and patient access programs, ensuring wider adoption of its therapies.
Key Developments:
In June 2024, The FDA has approved a combination of adagrasib and cetuximab for KRAS G12C-mutated colorectal cancer treatment, marking a significant advancement in precision medicine for this specific genetic mutation.
In April 2024, India has launched its first homegrown CAR T-cell therapy, a groundbreaking immunotherapy that modifies a patient's immune cells to better recognize and attack cancer cells. The President of India hailed it as a beacon of "new hope" in the fight against cancer.
Market Scope
Metrics | Details | |
CAGR | 19.8% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Therapy Type | Gene Induced Immunotherapy, Oncolytic Virotherapy, Gene Transfer Therapy |
Indication | Breast Cancer, Brain Cancer, Lung Cancer, Pancreatic Cancer, Liver Cancer, Others | |
End User | Hospitals, Cancer Research Institutes, Biotechnology & Pharmaceutical Companies | |
Regions Covered | North America, Europe, Asia-Pacific, South America and the Middle East & Africa | |
The global cancer gene therapy market report delivers a detailed analysis with 62 key tables, more than 57visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.