Bladder Cancer Treatment Market Size
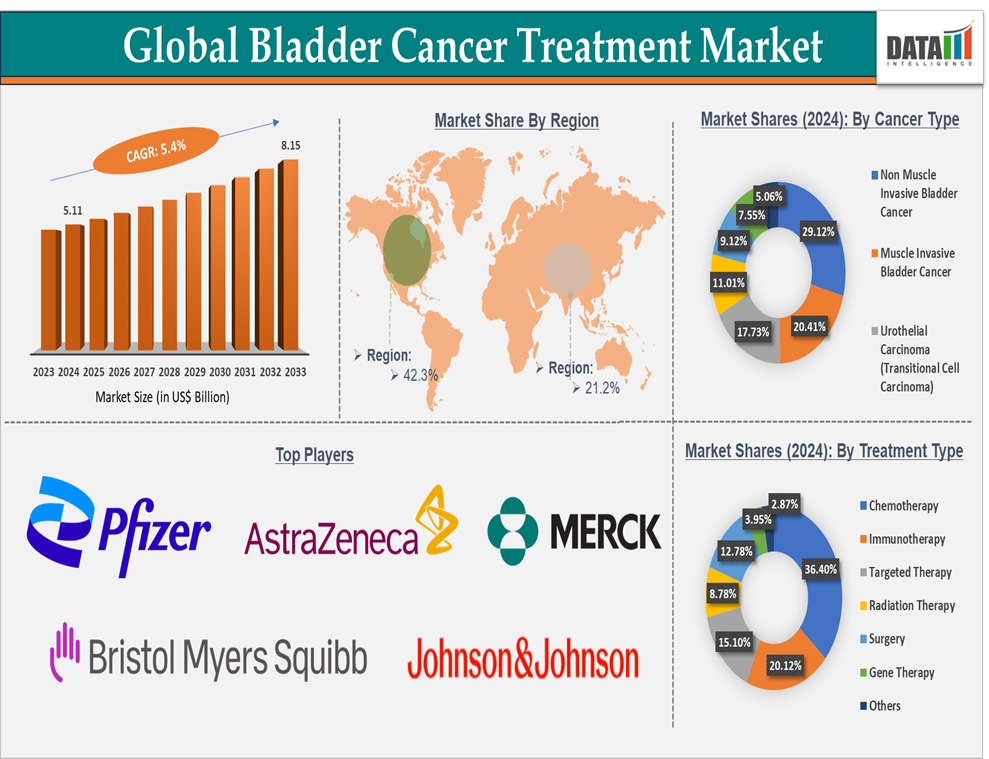
The global bladder cancer treatment market size reached US$ 5.11 Billion in 2024 and is expected to reach US$ 8.15 Billion by 2033, growing at a CAGR of 5.4% during the forecast period 2025-2033.
Bladder Cancer Treatment Market Overview
The bladder cancer treatment market is poised for significant growth, driven by advancements in treatment modalities, emerging therapies, and expanding access in developing regions. Continued innovation and investment in research and development are essential to address the evolving needs of bladder cancer patients worldwide. Ongoing research and development efforts are expected to yield more effective and less invasive treatment options, improving patient outcomes and quality of life.
Major players in the bladder cancer treatment market include Pfizer Inc., AstraZeneca, Merck & Co., Inc., Bristol-Myers Squibb Company, Johnson & Johnson, Merck KGaA, ImmunityBio, Inc., Ferring Pharmaceuticals Inc., Amneal Pharmaceuticals LLC, Astellas Pharma Inc., and UroGen Pharma, Inc. These companies are focusing on strategic initiatives such as partnerships, mergers, acquisitions, and new product launches to strengthen their market position .
Bladder Cancer Treatment Market Executive Summary
Bladder Cancer Treatment Market Dynamics: Drivers & Restraints
Novel product launches are significantly driving the market growth
Novel product launches are significantly driving the bladder cancer market growth by introducing more effective, targeted, and less invasive treatment options. These innovations improve patient outcomes, expand the range of treatment options available, and cater to unmet needs in the bladder cancer treatment landscape. Thus, major and emerging market players are focusing on novel therapeutics for better treatment of bladder cancer, which is further boosting the market growth.
For instance, in March 2025, AstraZeneca announced that the Imfinzi (durvalumab) in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by Imfinzi as adjuvant monotherapy after radical cystectomy (surgery to remove the bladder) approved in the US by the Food and Drug Administration (FDA) after securing Priority Review and was based on results from the NIAGARA Phase III trial for the treatment of adult patients with muscle-invasive bladder cancer (MIBC). These novel product launches drive the bladder cancer market by addressing unmet medical needs, improving survival rates, and providing patients with more personalized treatment options.
Nadofaragene Firadenovec (Adstiladrin) is an FDA-approved gene therapy for non-muscle invasive bladder cancer (NMIBC) that has shown promising results in patients who are unresponsive to Bacillus Calmette-Guérin (BCG) therapy. This treatment delivers the gene for interferon-alpha to the bladder, stimulating an immune response to attack cancer cells. Its approval has added a critical treatment option for patients who previously had limited alternatives. The success of such products is contributing to the shift towards personalized and immunotherapy-based treatments in bladder cancer, driving market growth.
Erdafitinib (Balversa) is FDA-approved for treating muscle-invasive bladder cancer (MIBC) with specific genetic mutations (FGFR3 or FGFR2 alterations). This targeted therapy blocks the fibroblast growth factor receptor, which is crucial for cancer cell growth. Balversa's launch opened a new pathway for patients with FGFR mutations who previously had limited treatment options. The approval of such targeted therapies contributes to a more refined treatment approach, particularly for specific genetic subsets of patients.
Limited efficacy in advanced stages are hampering the market growth
Bladder cancer is often diagnosed at a late stage, particularly in Stage III (locally advanced) or Stage IV (metastatic) forms, which poses significant treatment challenges. At these stages, the cancer may have spread beyond the bladder to nearby tissues or distant organs, making it harder to treat effectively with standard therapies. For instance, chemotherapy is commonly used for advanced bladder cancer, but its efficacy tends to decrease significantly as the disease progresses. Advanced-stage bladder cancer often shows chemoresistance, meaning the cancer cells become less responsive to the drugs, resulting in poor patient outcomes.
In advanced-stage bladder cancer, while cisplatin-based chemotherapy has been the standard treatment, the response rate in patients with metastatic disease is only around 40-50%, and the response duration is relatively short. This highlights the limited ability of traditional chemotherapy to provide long-term control over the disease in advanced stages. Similarly, immunotherapies like checkpoint inhibitors (e.g., atezolizumab, pembrolizumab) have shown promise in advanced bladder cancer treatment, but the response rates are still moderate, with some patients failing to respond or experiencing only temporary benefits.
For more details on this report – Request for Sample
Bladder Cancer Treatment Market, Epidemiology Analysis
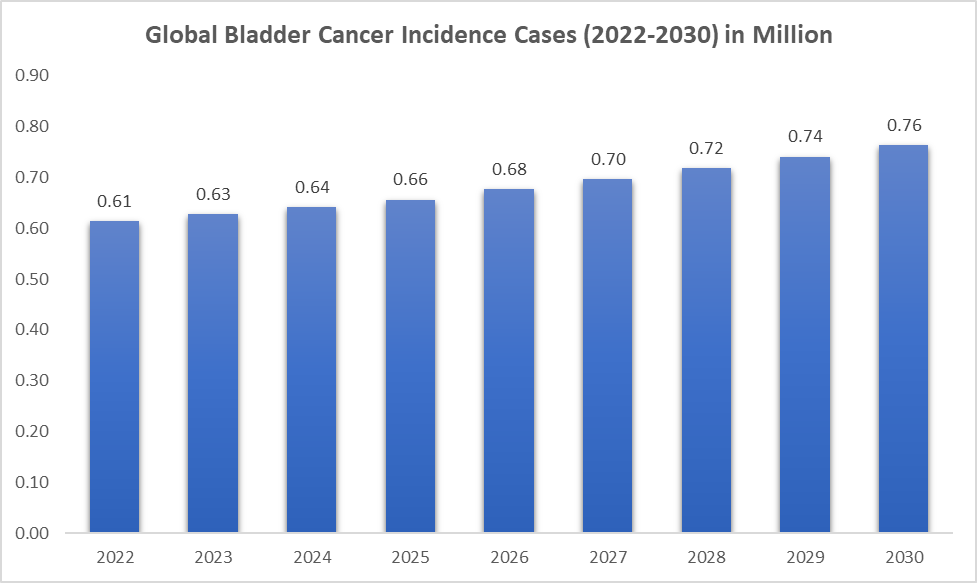
The incidence of bladder cancer is rising. WHO and DataM intelligence estimates that nearly 0.66 million incidence cases will occur worldwide in 2025. The epidemiology of bladder cancer significantly influences market growth by dictating the demand for specific treatment modalities, based on factors like age, gender, geographic location, and subtype. The rising incidence of bladder cancer, combined with the aging population and changing lifestyle factors, will continue to drive the demand for both traditional and innovative treatment options, pushing the market toward sustained growth.
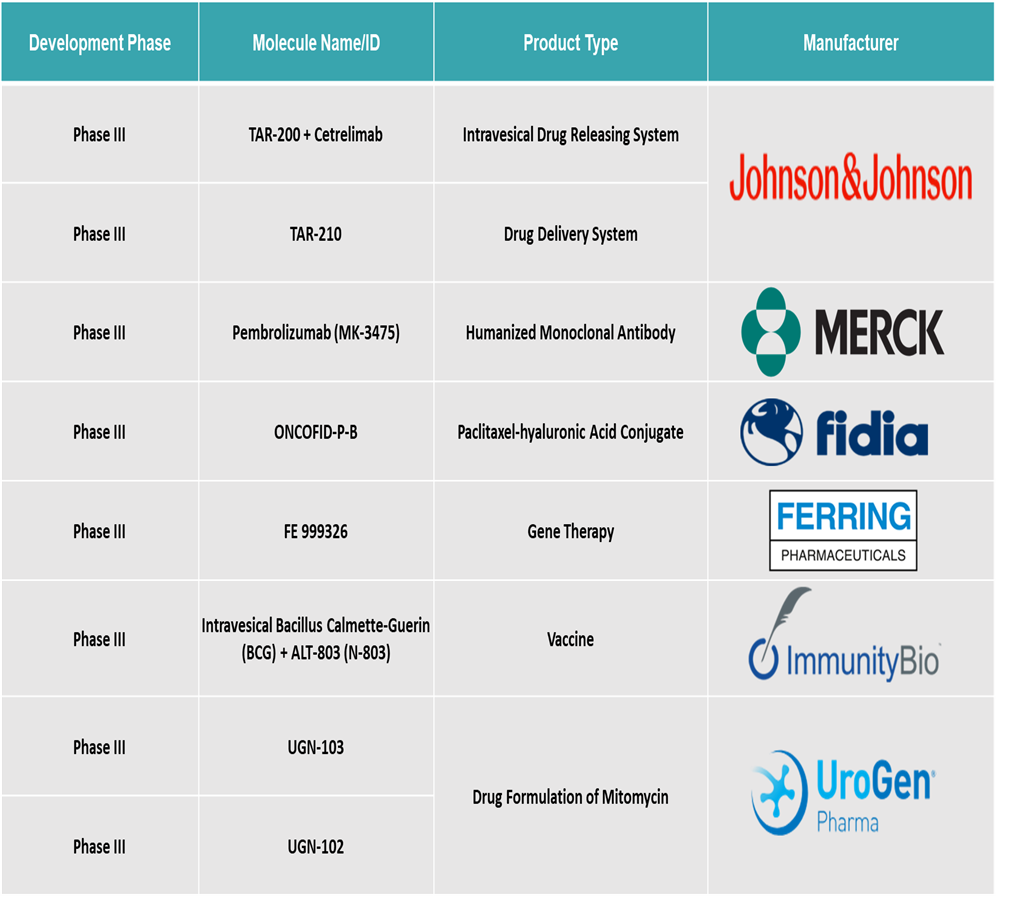
Bladder Cancer Treatment Market Pipeline Analysis
Top phase III pipeline products for bladder cancer:
Bladder Cancer Treatment Market, Segment Analysis
The global bladder cancer treatment market is segmented based on cancer type, treatment type, and region.
Treatment Type:
The chemotherapy segment is expected to hold 36.4% of the market share in 2024 in the bladder cancer treatment market
Chemotherapy plays a crucial role in the treatment of muscle-invasive bladder cancer (MIBC), which accounts for 20-30% of bladder cancer cases at diagnosis. Radical cystectomy (removal of the bladder) is often required for patients with MIBC, but chemotherapy is typically given neoadjuvantly (before surgery) or adjuvantly (after surgery) to improve outcomes. MVAC (Methotrexate, Vinblastine, Adriamycin, and Cisplatin) is a widely used chemotherapy regimen for patients with MIBC. MVAC has shown impressive results in improving survival rates when combined with cystectomy, making it a preferred therapy for patients with more advanced stages of bladder cancer.
In metastatic bladder cancer, where the disease has spread to distant organs, chemotherapy remains the primary treatment option for patients who are not candidates for surgery or other more advanced therapies. Chemotherapy regimens such as Cisplatin-based therapies are still considered the backbone of first-line treatment in metastatic cases. These regimens aim to control tumor growth, relieve symptoms, and improve overall survival.
While intravesical therapies like BCG (Bacillus Calmette-Guérin) remain the standard for Non-Muscle Invasive Bladder Cancer (NMIBC), chemotherapy is often used when BCG therapy is ineffective or unavailable. Chemotherapy agents such as Mitomycin C and Epirubicin are commonly delivered intravesically to treat NMIBC.
Bladder Cancer Treatment Market, Geographical Analysis
North America is expected to hold 42.3% of the global bladder cancer treatment market
North America is home to several leading pharmaceutical and biotechnology companies, such as Pfizer Inc., ImmunityBio, Bristol Myers Squibb, Merck, AstraZeneca, Johnson & Johnson, and other emerging players, which are heavily involved in developing and marketing bladder cancer therapies. These companies have extensive research programs focused on bladder cancer, driving the development of both chemotherapy agents and innovative immunotherapies. Their strong presence in the North American market boosts the availability and adoption of the latest treatments.
For instance, in April 2024, ImmunityBio, Inc. announced that the U.S. Food and Drug Administration (FDA) approved ANKTIVA (N-803, or nogapendekin alfa inbakicept-pmln) plus Bacillus Calmette-Guérin (BCG) for the treatment of patients with BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors. ANKTIVA, a first-in-class IL-15 agonist immunotherapy for NMIBC, received Breakthrough Therapy Designation and approval from the FDA based on the safety and efficacy outcome of complete responses (CR) and duration of complete response. With this novel product launches, North America hosts a large share of market, making cutting-edge therapies available to patients earlier than in other regions with early FDA approval.
Bladder Cancer Treatment Market Competitive Landscape
Top companies in the bladder cancer treatment market include Pfizer Inc., AstraZeneca, Merck & Co., Inc., Bristol-Myers Squibb Company, Johnson & Johnson, Merck KGaA, ImmunityBio, Inc., Ferring Pharmaceuticals Inc., Amneal Pharmaceuticals LLC, Astellas Pharma Inc., UroGen Pharma, Inc., Endo USA, and among others.
Bladder Cancer Treatment Market Scope
| Metrics | Details | |
| CAGR | 5.4% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Cancer Type | Non-Muscle Invasive Bladder Cancer, Muscle Invasive Bladder Cancer, Urothelial Carcinoma (Transitional Cell Carcinoma), Squamous Cell Carcinoma, Adenocarcinoma, Small Cell Carcinoma, and Sarcoma |
| Treatment Type | Chemotherapy, Immunotherapy, Targeted Therapy, Radiation Therapy, Surgery, Gene Therapy, and Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global bladder cancer treatment market report delivers a detailed analysis with 60 key tables, more than 52 visually impactful figures, and 167 pages of expert insights, providing a complete view of the market landscape.