Inflammatory bowel diseases (IBD) including Crohn’s and Ulcerative Colitis are chronic inflammatory conditions, affecting the gastrointestinal tract. Crohn’s disease can affect any part of the GI tract from mouth to anus and it majorly affects the ileum. Ulcerative colitis primarily affects the large intestine (colon) and rectum.
Causes:
The cause of IBD is unknown, but the primary factors are, environmental triggers, immune system disturbances genetic predispositions.
Symptoms:
Some common symptoms of IBD include fever, diarrhea, abdominal pain, rectal bleeding (more in UC) and weight loss, Fatigue, Irregular bowel movements, Sensation of incomplete stool evacuation.
How to Diagnose?
- Endoscopy (colonoscopy, sigmoidoscopy)
- Laboratory tests (Blood and Stool tests)
- Radiology tests (CT & MRI)
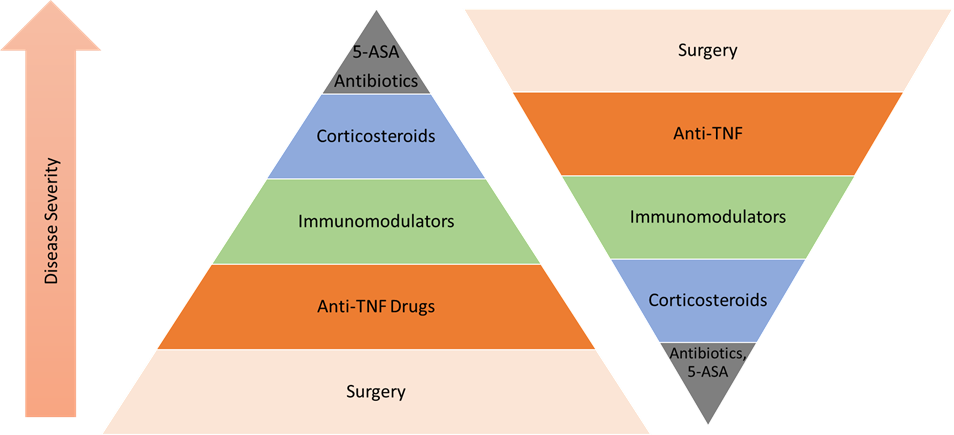
Treatment of Inflammatory Bowel Disease:
The IBD treatment algorithm depends on the stage of the disease. Combination therapy provides good remission rates. Biologics are the most promising therapeutic option for IBD in the current scenario.
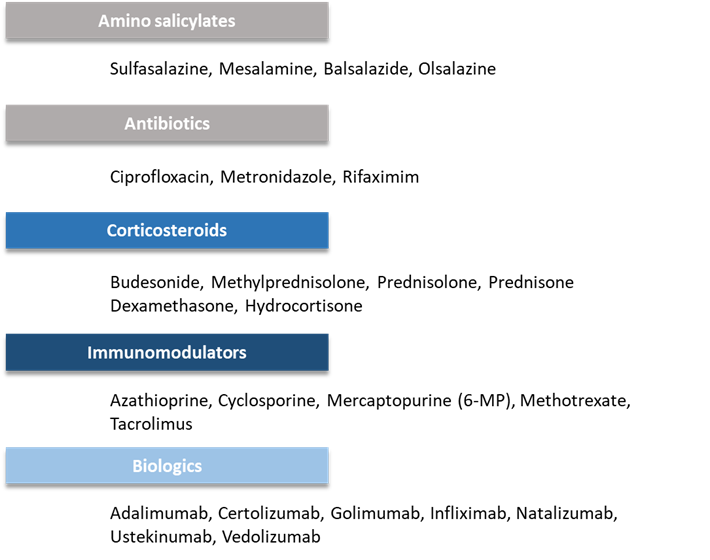
The common drugs in each category include the following:
Epidemiology of inflammatory bowel disease.
Globally approximately 4.84 million prevalent cases were reported in 2023, and by 2030, nearly 5.43 million cases are expected to be reported.
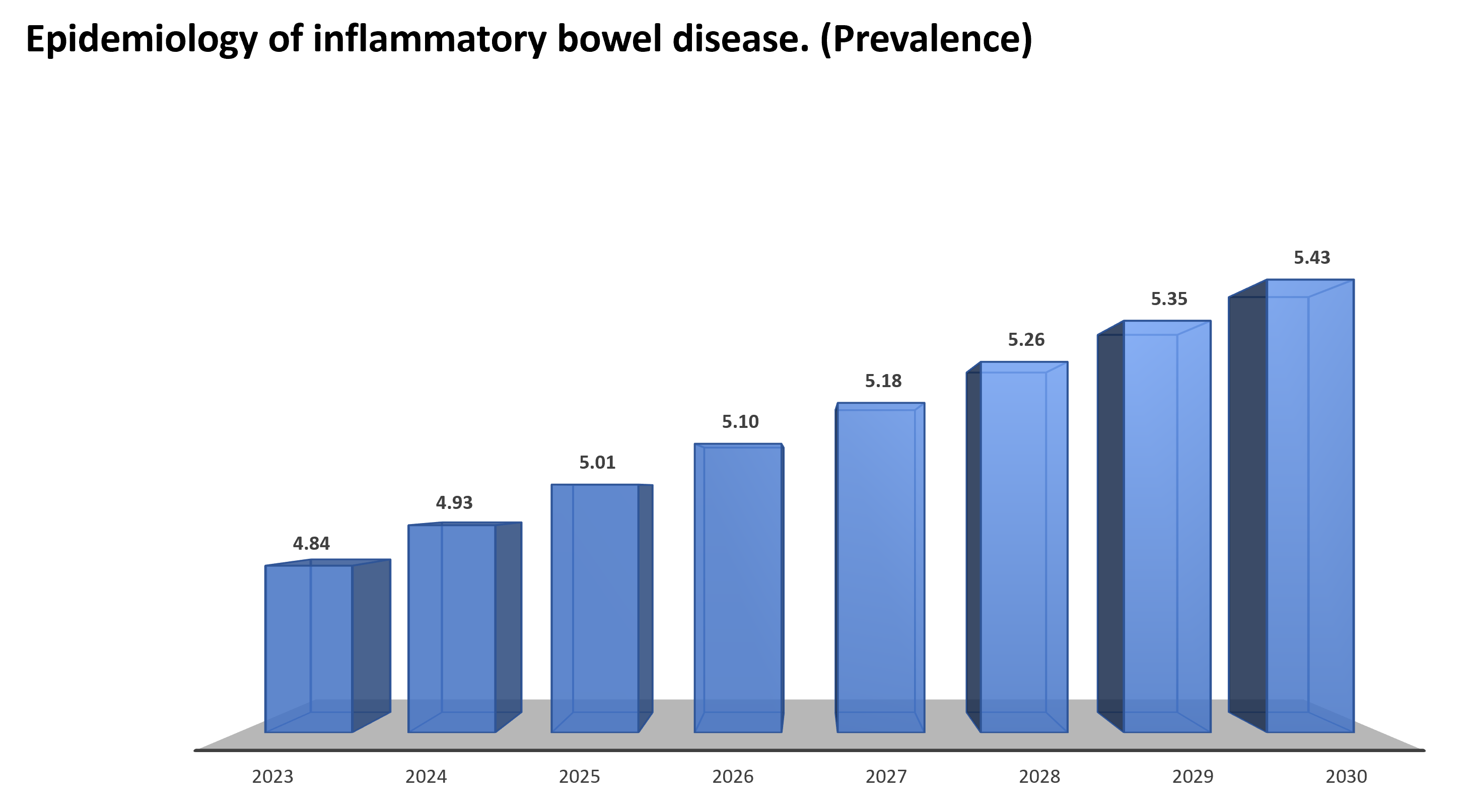
Global prevalence forecasts of inflammatory bowel disease (2023-2030)
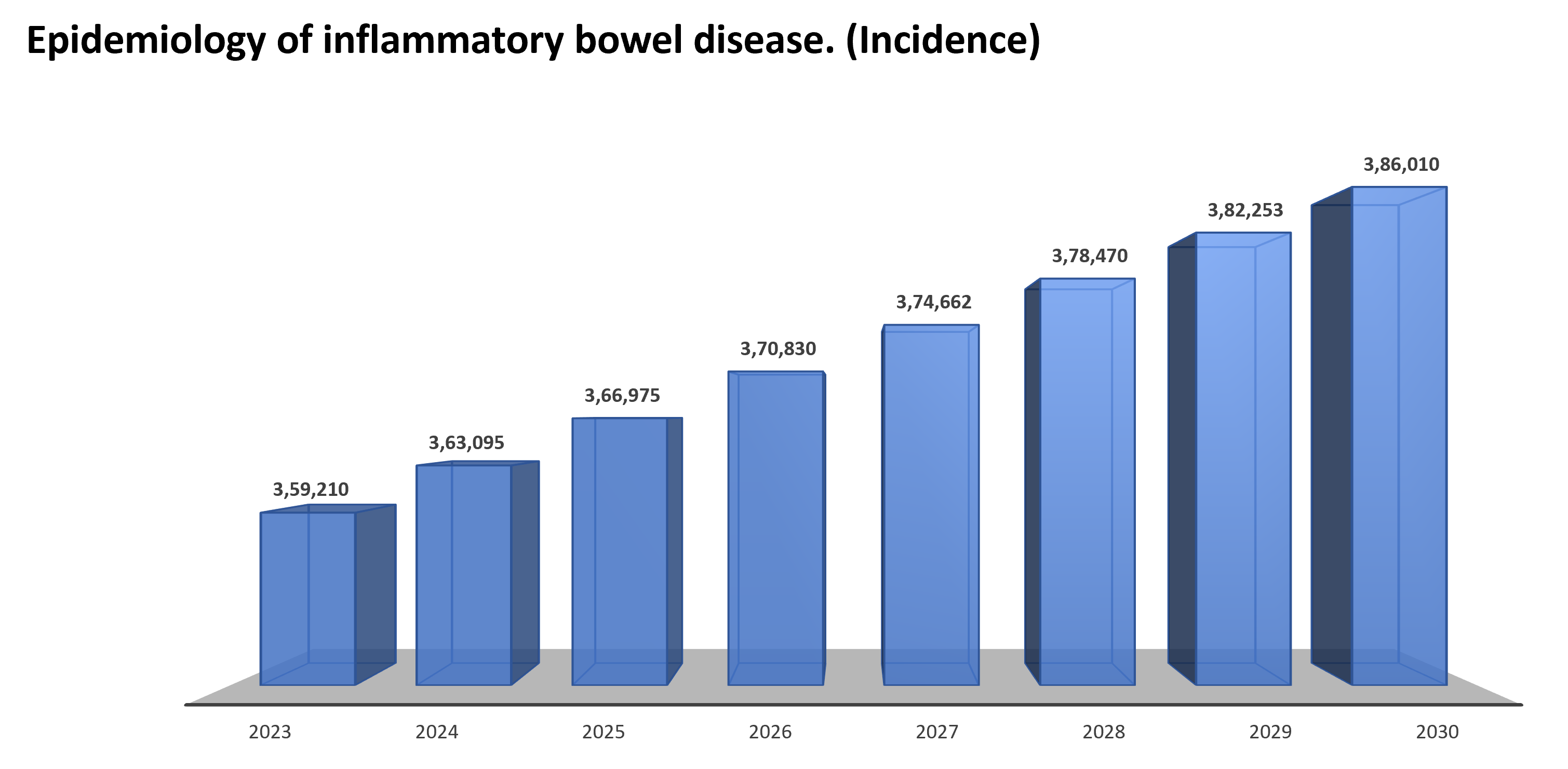
Global incidence forecasts of inflammatory bowel disease (2023-2030)
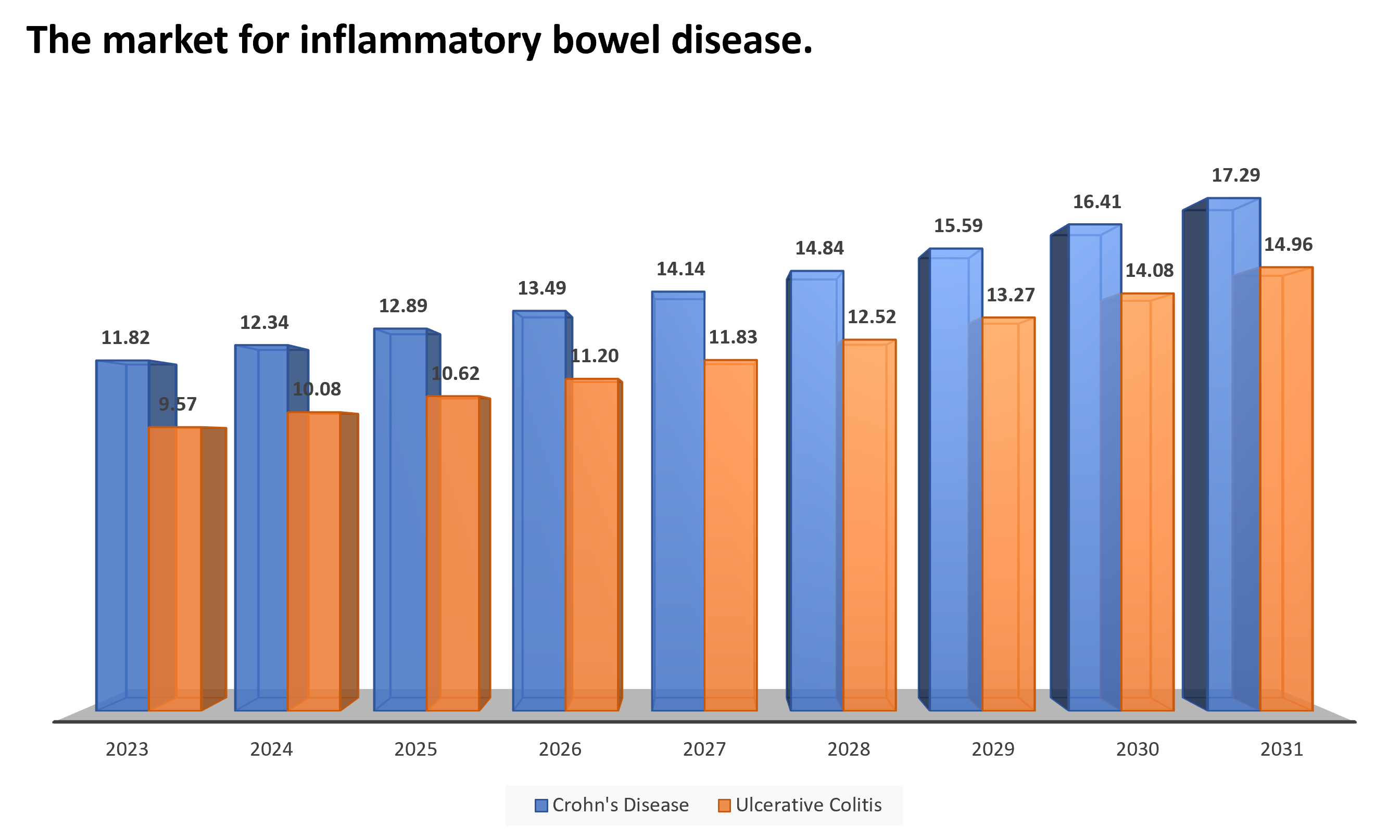
The market for inflammatory bowel disease.
The global Inflammatory bowel disease market was valued at $21.39 billion in 2023 and is anticipated to be valued at US$ XX million by 2031, registering a CAGR of 5%-6% over the forecast period. Crohn’s disease accounted for a major market share of ~55% which is nearly $12 billion in 2023
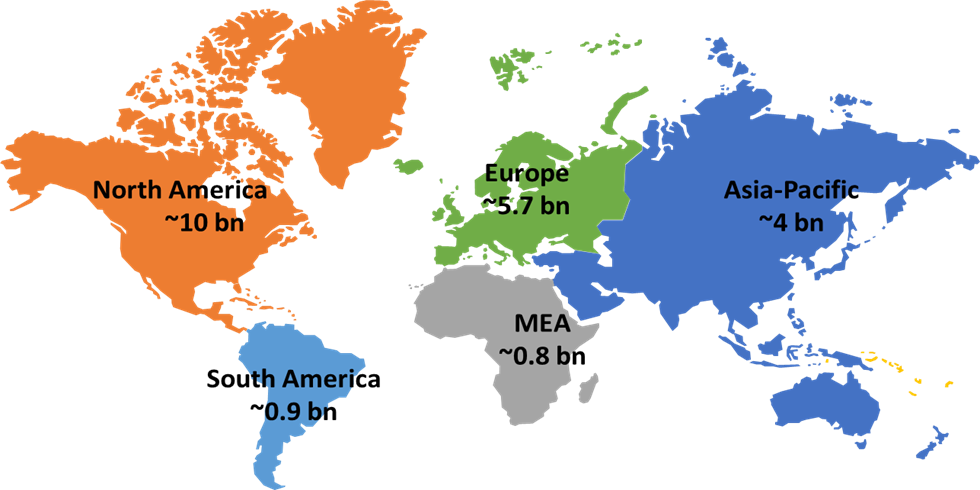
Inflammatory bowel disease market by geography
In 2023, North America dominates the inflammatory bowel disease treatment market with a share of ~46%, followed by Europe at ~27%, Asia-Pacific at ~18.5%, South America at ~4.3%, and the Middle East and Africa at ~3.8%
Key emerging therapies for inflammatory bowel disease
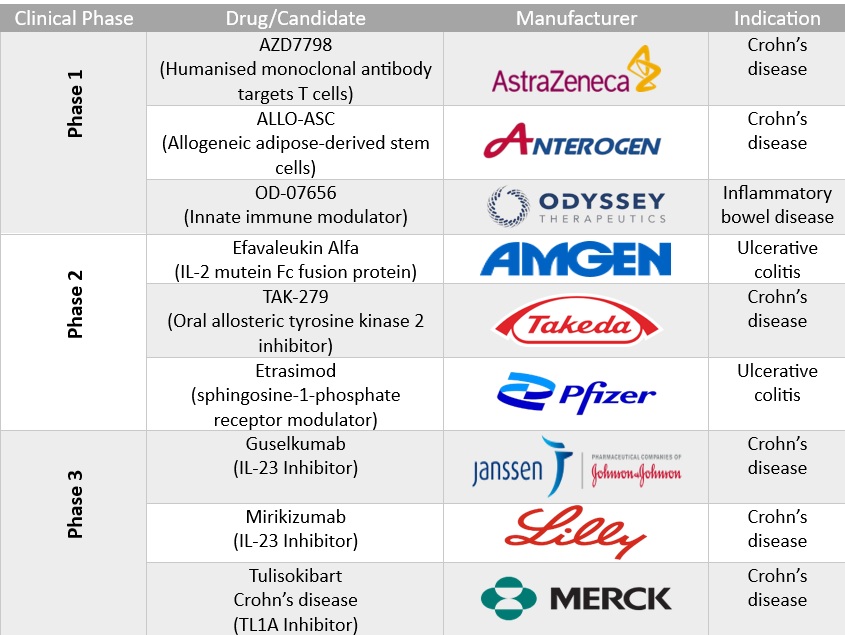
New IBD drug approvals anticipated in 2025:

Mirikizumab (Omvoh)
Omvoh by Eli Lilly and Company is anticipated to make a market entry in 2025 for the extended indication i.e, Crohn’s disease. The monoclonal antibody has received approval in the U.S., European Union, Japan, and other 44 countries for Ulcerative Colitis, and recently on December 13, 2024, the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) expressed a positive opinion for use in Crohn’s disease. The positive opinion was issued based on the phase 3 VIVID-1 trial that evaluated the safety and efficacy of mirikizumab in patients with or without prior biologic failure. Including the European Union, Lilly has submitted applications for marketing approval in the U.S., Japan, and the rest of the world. As per DataM estimates, Omvoh is anticipated to make a market entry for Crohn’s disease in Q3-Q4 of 2025.
Guselkumab (Tremfya)
Tremfya by Johnson & Johnson Services, Inc. is the first IL-23 inhibitor approved by the U.S. FDA for the treatment of Ulcerative Colitis. The monoclonal antibody is under the regulatory review process by the U.S. FDA. And EMA. The drug has produced positive results in phase 2/3 GALAXI clinical trials that have evaluated the efficacy and safety of guselkumab in Cronh’s disease patients, using the active drug control-Ustekinumab. The data suggested that guselkumab is superior to Ustekinumab upon endoscopic observations. As per dataM estimates, Tremfya is anticipated to receive a positive nod from the U.S. FDA and EMA by Q1, or early Q2, and make market entry latest by Q3 of 2025.
Recent Updates
- On September 24, 2024, The U.S. Food and Drug Administration (FDA) approved TREMFYA (guselkumab) for the treatment of moderate to severely active ulcerative colitis in adult patients. TREMFYA is a fully human, dual-acting monoclonal antibody targeting interleukin-23 and CD-64.
- On June 18, 2024, the U.S. Food and Drug Administration (FDA) approved SKYRIZI (risankizumab-rzaa) for the treatment of adult patients with moderately to severely active ulcerative colitis. Skyrizi is available for eligible, commercially insured patients with 0 out-of-pocket cost.
- On April 18, 2024, the U.S. Food and Drug Administration (FDA) approved ENTYVIO (vedolizumab) subcutaneous injection for maintenance therapy post i.v. Induction therapy with ENTYVIO in adults with moderately to severely active Crohn’s disease.