Radioligand Therapy Market Overview
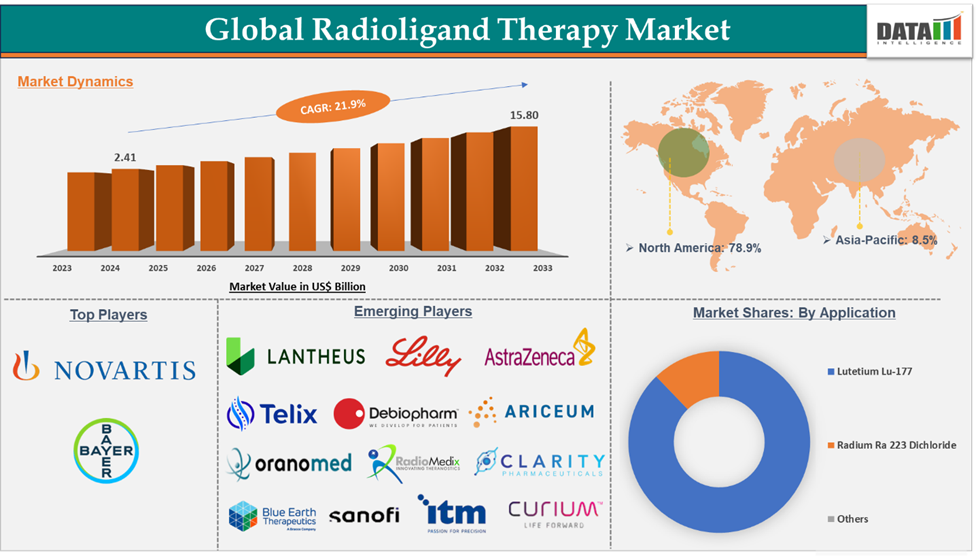
Radioligand Therapy Market reached US$ 2.41 billion in 2024 and is expected to reach US$ 15.80 billion by 2033, growing at a CAGR of 21.9% during the forecast period 2025-2033.
The radioligand therapy market is experiencing significant growth due to the rising incidence and prevalence of several types of cancers. Moreover, the rising demand for advanced targeted therapeutics like radioligand therapy, increasing research and development activities by key market players, advancements in clinical trials, and upcoming product approvals are the key factors that are expected to drive the market growth. The Expansion of RLT applications to other cancers is a key opportunity for market expansion in the forecast period.
Executive Summary
For more details on this report – Request for Sample
Radioligand Therapy Market Dynamics: Drivers & Restraints
The rising incidence and prevalence of cancer are driving the market growth
The rising incidence and prevalence of cancer mainly drive the global radioligand therapy market.
Radioligand therapy has evolved as a targeted treatment option for certain cancers, like prostate and neuroendocrine. With the rising incidence of this cancer, the adoption of radioligand therapy is expected to rise in the future. For instance, as per the GLOBOCAN projections, prostate cancer accounted for nearly 1.5 million cases in 2022, which is projected to reach 1.6 million in 2025 and 1.8 million in 2030.
RLT offers a precision approach by delivering radioactive isotopes directly to cancer cells, minimizing damage to healthy tissues, making it especially valuable in treating advanced-stage cancers such as neuroendocrine tumors and metastatic prostate cancer, which are becoming more prevalent.
Moreover, with the rising investments by emerging players and key market players, RLT is expected to expand to other prevalent cancers. Hence, the rising incidence and prevalence of cancers, growing patient population with unmet needs, and the anticipated market approvals can significantly boost the overall market growth in the forecast period.
High cost of radioligand therapies may restrain the market growth
The global radioligand therapy (RLT) market faces challenges due to the High Cost of RLTs. Radioligand therapy drugs that are currently approved include lutetium Lu 177 vipivotide tetraxetan, lutetium Lu 177 dotatate, and radium Ra 223 dichloride sold under the brand names Pluvicto, Lutathera, and Xofigo. These drugs, although life-changing, can be very expensive for patients with partial or no insurance coverage.
Pluvicto, which is indicated in prostate cancer, can cost up to US$ 50,000, and Lutathera, indicated for gastroenteropancreatic neuroendocrine tumors, can cost up to US$ 62,000. This can be a significant economic burden to patients with no insurance coverage.
Moreover, in India, prostate cancer cases are expected to account for 41,700 cases in 2025. However, the cost of Pluvicto is approximately INR 3.9 million, which is not affordable by the majority of the patient population. The same scenario is seen in low-income and emerging countries, where reimbursement opportunities are scarce.
Moreover, several new radioligand therapies are expected to make market entry in the forecast period, which are indeed expected to be expensive. Due to their high cost, the adoption rate is expected to be slower in the forecast period.
Radioligand Therapy Market Segment Analysis
The global radioligand therapy market is segmented based on type, target, application, and region.
Lutetium Lu 177 in the type segment accounted for 87.8% of the market share in 2024 in the global radioligand therapy market.
Lutetium Lu 177 is a widely used radionucleotide in treating cancers such as prostate cancer and neuroendocrine tumors (NETs), due to its ability to deliver precise radiation to cancer cells while minimizing damage to surrounding healthy tissue. At present, three radioligand therapies are approved and available in the market, of which two therapies have leutitium Lu 177 radionucleotide as the key therapeutic moiety. These products include Pluvicto (lutetium Lu 177 vipivotide tetraxetan) and Lutathera (lutetium Lu 177 dotatate) developed and manufactured by Novartis AG. At present, these radioligand therapies are the top-selling products with the highest market share, generating a combined 2.1 billion in 2024.
Radioligand Therapy Market Geographical Analysis
North America dominated the radioligand therapy market with the highest share of 78.9% in 2024
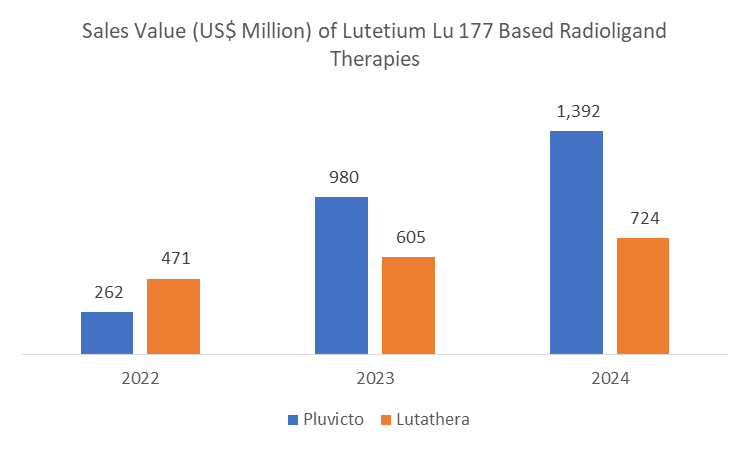
In 2024, North America led the global radioligand therapy market, mainly due to the significant revenue generated by key market players from the region. The United States, in particular, contributed the majority of this growth, reflecting its advanced healthcare infrastructure, widespread adoption of radioligand therapy, and a pharmaceutical industry. For instance, in the radioligand therapy market, Novartis AG is a key market player with its product Pluvicto generating US$ 1,392 million globally, of which $1,157 million (83%) came from the U.S. market alone. Similarly, Lutathera generated US$ 724 million globally, with US$ 513 million (71%) from the U.S.
Radioligand Therapy | Sales (US$ Million) in 2024 | U.S. Sales | U.S. Share |
Pluvicto | 1,392.00 | 1,157.00 | 83% |
Lutathera | 724.00 | 513.00 | 71% |
These figures highlight the strong demand, rapid clinical adoption in the region, which have all played a pivotal role in North America's market dominance.
Radioligand Therapy Market Major Players
The major players in the radioligand therapy market are Novartis AG and Bayer AG.
Radioligand Therapy Market Emerging Players
The emerging players in the radioligand therapy market are Eli Lilly and Company., Lantheus, Curium, AstraZeneca, ITM Isotope Technologies Munich SE, Telix Pharmaceuticals Limited, Oranomed, Sanofi, RadioMedix, Inc., Clarity Pharmaceuticals, Ariceum Therapeutics, and Blue Earth Therapeutics, among others.
Radioligand Therapy Market Key Developments
In May 2025, Blue Earth Therapeutics announced the enrollment of the first two patients in a Phase 2 clinical trial for its radio hybrid, lutetium-labelled, PSMA-targeted investigational radioligand therapy. The primary measure of efficacy will be the proportion of patients achieving a ≥50% reduction in PSA levels, as well as assessing radiographic progression-free survival and patient safety.
In March 2025, Novartis AG announced that the U.S. Food and Drug Administration (FDA) approved the extended application of Pluvicto for earlier use before chemotherapy in PSMA-positive metastatic castration-resistant prostate cancer. With this approval, the target patient population of Pluvicto is expected to triple.
Market Scope
Metrics | Details | |
CAGR | 21.9% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Type | Lutetium Lu-177, Radium Ra 223 Dichloride, Others |
Target | Prostate-specific Membrane Antigen (PSMA), Somatostatin Receptor (SSTR) | |
Application | Prostate Cancer, Gastroenteropancreatic Neuroendocrine Tumours (GEP-NETs), Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |