Prostate Cancer Market Size & Industry Outlook
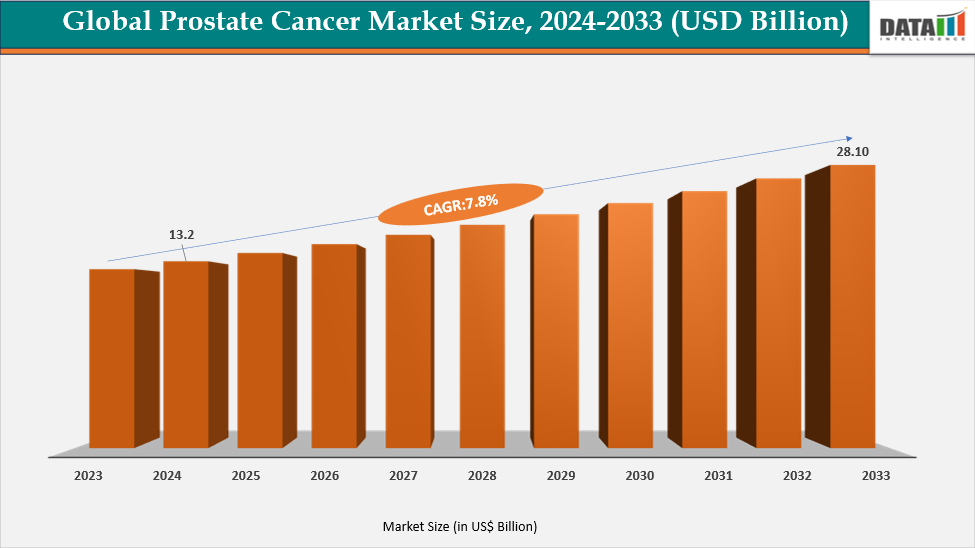
The global prostate cancer market is projected to grow from US$ 13.22 Billion in 2024 to US$ 28.10 Billion by 2033, registering a CAGR of 7.8% during the forecast period. The global market for prostate cancer is expanding due to rising prevalence and demand for advanced therapies, early diagnostic solutions, and supportive care options. Pharmaceutical manufacturers are investing heavily in research and development to strengthen oncology portfolios and address unmet medical needs. Prostate cancer is the most common cancer among men in the United States, and the incidence of advanced disease is increasing rapidly. It is one of the most common cancers in men. It is usually seen in men after the age of 50 years, but the risk increases with increasing age.
For instance, according to Cancer Therapy Advisor in 2024, there are 299,010 new instances of prostate cancer identified in the United States, making up 14.9% of all new cancer cases. The annual rate of new cases of prostate cancer, based on age-adjusted cases from 2017 to 2021, is 116.5 per 100,000 males.
Key Highlights
Hormonal therapy is the first-line treatment for prostate cancer: it is capturing 42.1% share in 2024.
North America leads the prostate cancer drug market, holding 40.1% share in 2024, driven by strong healthcare systems, better infrastructure and active clinical research backed by FDA approvals.
The oral route is the leading route of the administration segment, projected to grow at an 48.1%, as it provides comprehensive cancer care, advanced technologies, and direct access to branded therapies.
The major market players in the prostate cancer market are Bayer AG, Johnson & Johnson, Novartis Pharmaceuticals Corporation, Blue Earth Diagnostics, Inc., Telix Pharmaceuticals (US) Inc., Pfizer Inc., Astellas Pharma US, Inc., Sanofi-Aventis U.S. LLC., Genentech USA, Inc., Pfizer Inc., AstraZeneca, and Eli Lilly Inc., among others.
Market Dynamics
Drivers:Rising Prevalence of prostate cancer is significantly driving the prostate cancer market growth
The rising prevalence and risk of prostate cancer are increased by a number of factors: older age, race/ethnicity, family history, inherited gene changes, diet, obesity, smoking, chemical exposures, inflammation of the prostate, sexually transmitted infections, and vasectomy. Men under 40 are unlikely to develop prostate cancer, but after age 50, the risk increases significantly. Men over 65 account for about 6 out of 10 cases of prostate cancer.
For instance, according to the World Cancer Research Fund, Globally, 1,467,854 cases of prostate cancer were found IN 2022. In the United States had the highest number of prostate cancer cases in 2022 (230,125), followed by China (134,156) and Japan (104,318).
Restraints:High treatment costs and risks of therapy are hampering the growth of the prostate cancer market
The prostate cancer drug market faces challenges due to high treatment costs, particularly for targeted therapies and immunotherapies, which create affordability issues for patients and strain healthcare systems. Limited insurance coverage and disparities in access to advanced therapies also limit market penetration in low- and middle-income countries.
For instance, according to the American Cancer Society, the average cost of early-stage prostate cancer treatment would be less than $10,000, while the treatment cost for advanced prostate cancer may reach $50,000–$100,000 in the United States, and in Canada the average cost of treatment is $15,000 – $45,000 compared to the USA.
For more details on this report,Request for Sample
Prostate Cancer therapeutics market, Segment Analysis
The global prostate cancer therapeutics market is segmented based on therapy, stages of cancer, distribution channel, and region.
By Therapy:The hormonal therapy segment is dominating the prostate cancer market with a 42.1% share in 2024
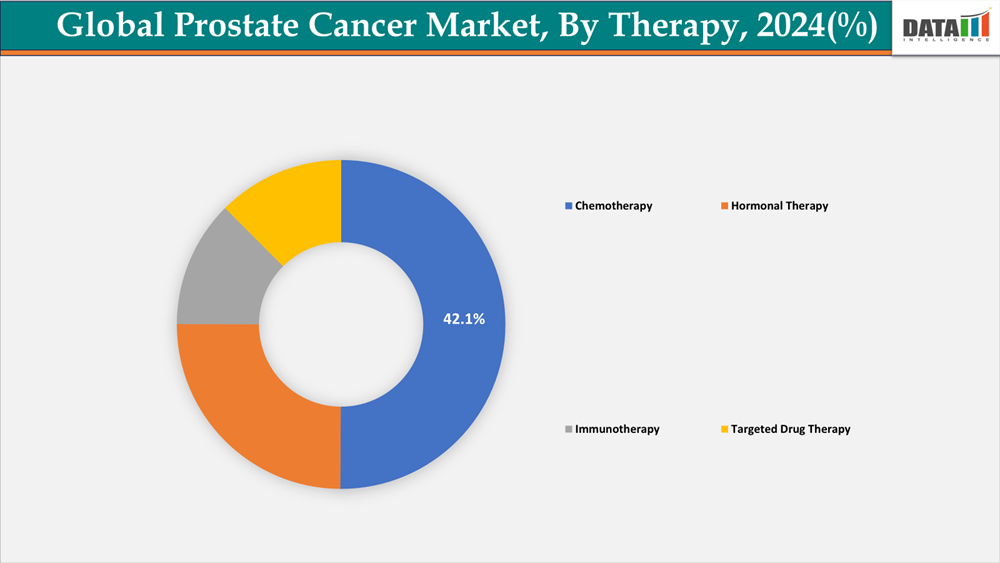
Prostate cancer usually depends on the hormone testosterone to grow. Hormone therapy blocks or lowers the amount of testosterone in the body. This can lower the risk of prostate cancer coming back when you have it with other treatments. The dominance is because the efficiency of LHRH agonists, antagonists, and anti-androgens in regulating the course of disease has been credited with this supremacy. The use of hormonal medications in first-line treatment regimens has strengthened preference for them. Wider acceptance has been aided by the growing use of combination regimens and positive clinical trial results. These treatments have enabled long-term disease control, particularly in cases of metastatic and advanced prostate cancer.
For instance, in November 2023, the FDA approved enzalutamide for non-metastatic castration-sensitive prostate cancer (nmCSPC) with biochemical recurrence at high risk for metastasis (high-risk BCR); it is an androgen receptor inhibitor. In addition to the rise in prostate cancer therapies, Xtandi is one of the most commonly used medications since patients are taking it for longer periods of time, an average of nine months.
By Route of Administration – Oral route is growing in the market with strong growth potential with a 48.7% market share in 2024
The trend is shifting towards the oral route of administration because maintaining a normal routine as much as possible provides a psychological benefit to some patients. They are able to keep their sense of normalcy by taking drug therapy at home. Daily oral therapy may potentially alter how a patient experiences treatment side effects. Compared to IV therapy, some patients experience more consistent but milder side effects.
Agents that are taken orally are essential in the treatment of prostate cancer because they give patients a practical and affordable choice. Nevertheless, they are also linked to problems with adherence, which may jeopardize the effectiveness of treatment. Patients with prostate cancer nowadays frequently receive combination treatments. Oral ADT simplifies treatment regimens when patients are already managing other oral medications like abiraterone or enzalutamide.
Geographical Analysis
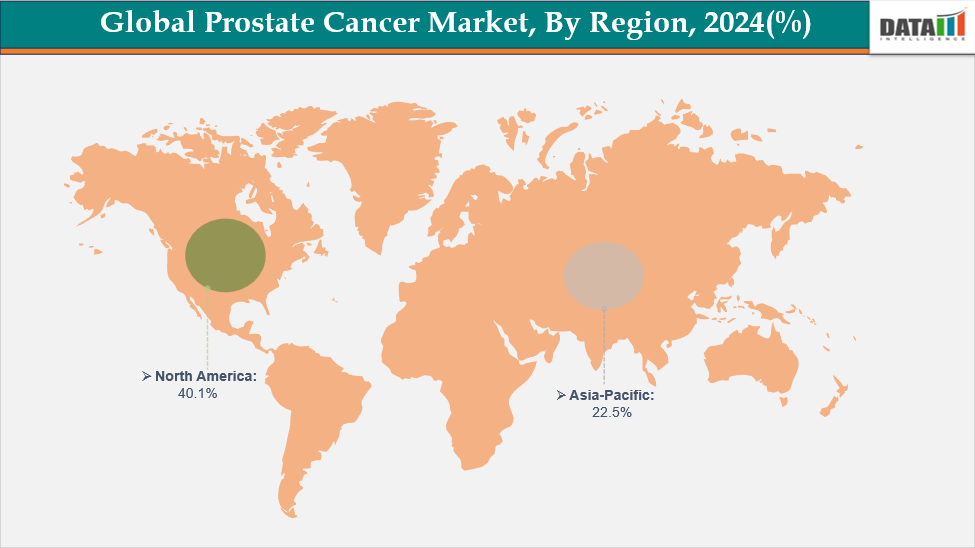
North America is expected to dominate the global prostate cancer therapeutic market with 40.1% share in 2025
North America has a high market growth for prostate cancer drugs due to the high prevalence of prostate cancer, an aging population, a well-established advanced healthcare infrastructure, substantial R&D investments, early adoption of cutting-edge therapies like androgen receptor inhibitors, supportive regulatory frameworks, and robust reimbursement policies that encourage access to new treatments. These are some of the factors contributing to North America's strong prostate cancer drug market growth. Effective therapies are in high demand due to these features, which also make it easier to develop and market new medications.
For instance, according to the American Cancer Society, in 2025, about 313,780 new cases of prostate cancer and about 35,770 deaths from prostate cancer are expected in the United States, and about 1 in 8 men will be diagnosed with prostate cancer during their lifetime.
Europe is expected to grow in the global prostate cancer therapeutic market share with 35.1% in 2024
Prostate cancer cases are increasing in Europe mainly due to an aging population, as risk rises sharply after age 50. Expanded PSA screening and improved cancer registries detect more cases, including indolent tumors, driving higher incidence. Greater awareness, genetic testing, and early detection add to reported numbers. Lifestyle changes such as obesity, sedentary habits, alcohol use, and Western diets further elevate risk. While incidence rises, mortality is more stable, with survival improving.
For instance, according to the latest EUROCARE-6 statistics on cancer prevalence, around one in 20 people in Europe have faced a cancer diagnosis in their lifetime. Among these, breast and prostate cancers are the most common, with prostate cancer representing a striking 40% of all male cancer survivors. Across the 29 European countries included in the study, an estimated 23.7 million people out of 477.9 million inhabitants are living with or have experienced cancer, underscoring the significant public health burden across the continent.
The Asia Pacific region is the fastest-growing region in the prostate cancer therapeutic market, with 22.5% share in 2025.
The Asia Pacific prostate cancer market is experiencing rapid growth due to demographic changes and improved healthcare access. Rising cancer incidence, urbanization, and lifestyle changes are driving demand for oncology treatments. Governments in China, India, and Japan are investing in conducting awareness programs, building healthcare infrastructure, and promoting advanced treatments. The expansion of generic and biosimilar drugs is making therapies more affordable and accessible.
For instance, in May 2025, according to the Times of India, the Government of Kerala launched the special cancer screening named ‘Aarogyam Anadam – Akattam Arbudam’ public cancer campaign, aimed at cancer prevention awareness and treatment. It was held on World Cancer Day, and screenings conducted for approx. 1.55 million people have been done so far, and 242 cancer cases have been detected.
Competitive Landscape
Top companies in the prostate cancer market are Bayer AG, Johnson & Johnson, Novartis Pharmaceuticals Corporation, Blue Earth Diagnostics, Inc., Telix Pharmaceuticals (US) Inc., Pfizer Inc., Astellas Pharma US, Inc., Sanofi-Aventis U.S. LLC., Genentech USA, Inc., Pfizer Inc., AstraZeneca, and Eli Lilly Inc., among others.
Bayer AG: Bayer AG is a leading player in the prostate cancer market; their strong oncology pipelines and portfolios in chemotherapy, targeted therapy, and immunotherapy drive market growth. Innovative treatments, FDA approvals, and strategic collaborations further support market growth. Mergers and acquisitions further strengthen their drug pipelines.
Market Scope
Metrics | Details | |
CAGR | 7.8% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | By Therapy | Chemotherapy, Hormonal Therapy, Immunotherapy, Targeted Therapy |
By Routes of Administration | By Oral Route, By Intravenous (IV) Route, Other routes | |
By Distribution Channel | Hospital Pharmacy, Retail Pharmacy, Online Pharmacies | |
Regions Covered | North America, Europe, Asia-Pacific, South America and the Middle East & Africa | |
The global prostate cancer market report delivers a detailed analysis with 56 key tables, more than 52 visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more pharmaceutical-related reports, please click here