Global Phenylketonuria Treatment Market – Industry Trends & Outlook
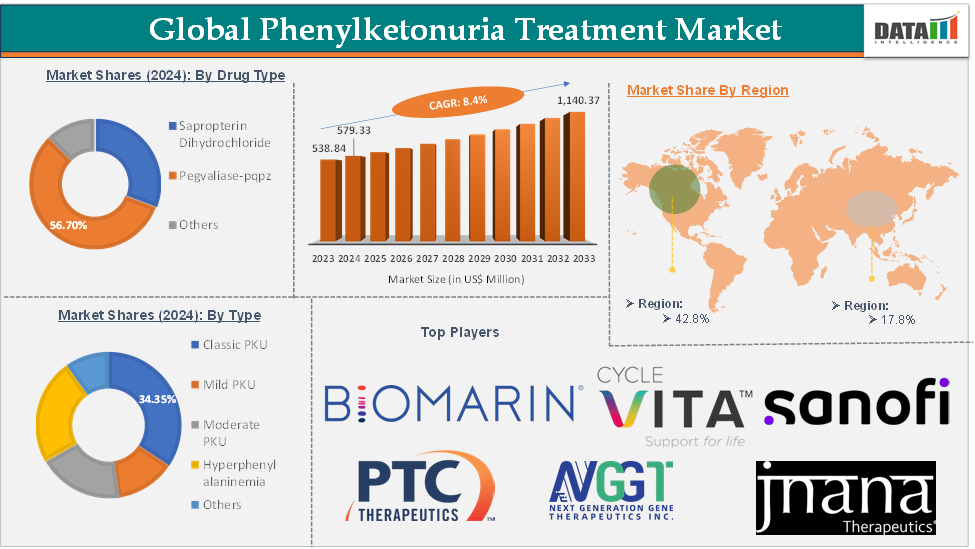
The global phenylketonuria treatment market was valued at US$ 538.84 Million in 2023. The market size reached US$ 579.33 Million in 2024 and is expected to reach US$ 1,140.37 Million by 2033, growing at a CAGR of 8.4% during the forecast period 2025-2033.
Phenylketonuria (PKU) is a rare, inherited metabolic disorder in which the body is unable to properly break down the amino acid phenylalanine (Phe), resulting in its toxic accumulation in the blood and brain. If untreated, PKU can lead to intellectual disability, seizures, behavioral problems, and other neurological complications.
The primary drivers of PKU are genetic mutations in the PAH gene, with over 300 identified variants that affect enzyme activity to varying degrees. Because PKU is inherited, family history and ancestry play a significant role in risk, with higher incidence among certain ethnic groups, such as those of European or Native American descent. Early detection through newborn screening and ongoing research into the genetics, biochemistry, and treatment of PKU also drives progress in understanding and managing the disorder.
Current trends in PKU management include advances in treatment options beyond the traditional strict low-phenylalanine diet. Newer medications, enzyme replacement therapies, and investigational gene therapies are expanding the possibilities for disease management and diet liberalization. Digital health solutions, such as AI-powered smartphone apps, are emerging to help patients track their dietary intake, monitor Phe levels, and manage treatment regimens more effectively.
Opportunities in the PKU field are numerous and promising. The development of novel therapies, including gene therapy, mRNA-based treatments, and small-molecule drugs, offers the potential for more effective and convenient management of the disorder. Expanding newborn screening programs globally and increasing awareness of PKU in underserved regions can improve early diagnosis and outcomes for more patients worldwide.
Global Phenylketonuria Treatment Market – Executive Summary
Global Phenylketonuria Treatment Market Dynamics: Drivers
Rising research and development (R&D) activities
The increasing research and development (R&D) activities are a major driver for the phenylketonuria (PKU) treatment market. This surge in R&D is fueled by the need for more effective, convenient, and long-term management solutions for PKU, which traditionally relies on strict dietary restrictions and specialized medical formulas. Pharmaceutical and biotechnology companies are investing heavily in developing new therapies, including enzyme replacement therapies, gene therapies, and small-molecule drugs, to address the limitations of current treatments and improve patient outcomes.
Collaborations among key industry players, academic institutions, and research organizations are accelerating the pace of innovation. These partnerships help combine expertise, share resources, and expedite the introduction of novel treatments into the market.
For instance, in March 2025, PTC Therapeutics, Inc. shared new data being presented from the Phase 3 APHENITY trial and subsequent open-label extension study at the 2025 American College of Medical Genetics and Genomics (ACMG) Annual Clinical Genetics Meeting. These data provide further evidence of the potential meaningful benefits of sepiapterin treatment for the full spectrum of phenylketonuria (PKU) patients, including significant diet liberalization.
Rising prevalence and early diagnosis
Rising prevalence and early diagnosis are important drivers for the growth of the PKU treatment market. While PKU is a rare genetic disorder, its reported prevalence has increased in certain regions due to improved diagnostic capabilities, expanded newborn screening programs, and greater awareness among healthcare professionals and the public.
Early diagnosis, primarily through routine newborn screening, is critical because it allows for prompt initiation of treatment. When PKU is detected early, patients can start a low-phenylalanine diet and other interventions immediately, which helps prevent the severe neurological and developmental complications associated with untreated PKU. This not only improves patient outcomes but also increases the number of individuals identified as needing lifelong management and specialized therapies.
As more cases are identified and diagnosed early, the demand for effective PKU treatments, supportive products (such as medical foods and supplements), and innovative therapies grows. This drives market expansion by enlarging the patient population and highlighting the need for continued research, development, and commercialization of new treatment options.
For instance, in October 2024, PhenylAde GMP ULTRA Plain is a new medical food formula developed specifically for the dietary management of Phenylketonuria (PKU), a genetic disorder where the body cannot break down the amino acid phenylalanine (Phe), requiring strict dietary control. This formula is intended for individuals over three years old and must be used under medical supervision.
Global Phenylketonuria Treatment Market Dynamics: Restraints
High treatment costs
High treatment costs are a significant restraint for the phenylketonuria (PKU) therapeutics market. The annual cost of PKU treatment can range from tens of thousands of dollars per patient, especially when factoring in specialized medical foods, enzyme replacement therapies, and novel pharmacological treatments. For example, in the United States, annual treatment costs can reach between US$ 19,057 and US$ 54,147 for some patients, with additional expenses for low-protein foods and ongoing monitoring.
These high costs limit patient access, particularly in regions with limited insurance coverage or government reimbursement programs. Many families face substantial financial burdens, and in developing countries, the expense can make treatment unaffordable for most patients. This restricts market growth by reducing the number of patients who can access advanced therapies and may also slow the adoption of new, innovative treatments.
Global Phenylketonuria Treatment Market Dynamics: Opportunities
Expansion of next-generation therapies
The expansion of next-generation therapies represents a major opportunity for the phenylketonuria (PKU) treatment market. This opportunity is driven by ongoing advancements in biotechnology and pharmaceutical innovation, which are resulting in a robust pipeline of novel treatments beyond traditional dietary management and enzyme replacement.
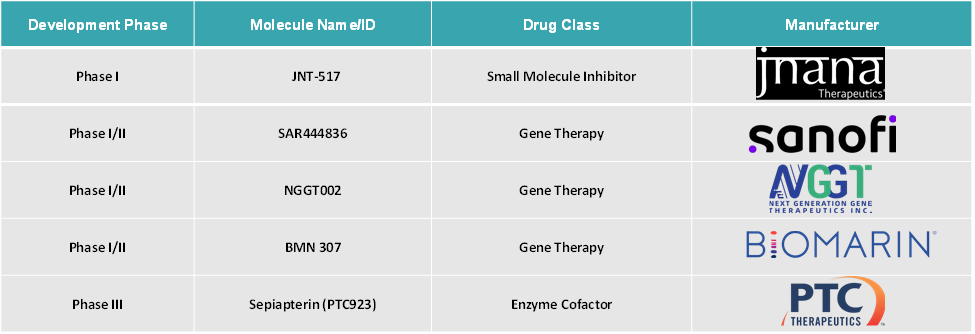
Next-generation therapies include a range of cutting-edge approaches, such as companies like Next Generation Gene Therapeutics (NGGT) are advancing gene therapy candidates such as NGGT002, which have shown early promise in clinical trials for providing durable reductions in phenylalanine levels and potentially offering a long-term or even curative solution for PKU.
Small-molecule inhibitor drugs like JNT-517, which inhibit the phenylalanine transporter SLC6A19, are being developed as oral treatments suitable for all ages and genotypes of PKU. These have demonstrated clinically meaningful reductions in plasma phenylalanine and favorable safety profiles in early studies.
Innovations such as Alltrna’s AP003, an engineered tRNA therapy, have shown the ability to restore protein production in preclinical models, offering another novel mechanism for treating PKU. Enhanced enzyme replacement therapies and better-tolerated medical foods are also part of the evolving landscape.
For more details on this report, Request for Sample
Global Phenylketonuria Treatment Market Dynamics: Pipeline Analysis
Global Phenylketonuria Treatment Market - Segment Analysis
The global phenylketonuria treatment market is segmented based on type, drug type, age group, route of administration, end-user, and region.
Drug Type:
The pegvaliase-pqpz drug type segment in the phenylketonuria treatment market was valued at US$ 328.48 Million in 2024
Pegvaliase-pqpz (palynziq) belongs to the enzyme substitution therapy segment of the phenylketonuria (PKU) treatment market. Enzyme substitution therapy involves the administration of a functional enzyme that can metabolize phenylalanine (Phe) in the body, compensating for the deficiency or absence of the patient’s phenylalanine hydroxylase (PAH) enzyme. Pegvaliase-pqpz is approved for use in adults with PKU who have uncontrolled blood phenylalanine concentrations despite existing dietary management. The drug is administered as a subcutaneous injection, making it suitable for individuals who struggle with strict dietary adherence or for whom other treatments have proven ineffective.
Its primary application is to reduce and maintain blood Phe levels within a safe range, thereby helping to prevent the neurological and cognitive complications associated with untreated or poorly controlled PKU. This therapy is particularly valuable for patients with severe or classic PKU, offering a new option for those who have limited response to other interventions.
The main drivers for the pegvaliase-pqpz drug type segment include the unmet medical need among adults with poorly controlled PKU, the limitations of traditional dietary management, and the desire for a more convenient and effective treatment option. Many patients find lifelong dietary restrictions challenging, and some do not respond adequately to other available therapies such as sapropterin.
The introduction of enzyme substitution therapy addresses these gaps by providing a mechanism to metabolize Phe directly, independent of the patient’s residual PAH activity. Additionally, ongoing research, regulatory approvals, and patient demand for improved quality of life continue to drive adoption and development within this segment.
Global Phenylketonuria Treatment Market – Geographical Analysis
North America phenylketonuria treatment market was valued at US$ 247.96 Million in 2024
North America benefits from well-established newborn screening programs that ensure the early detection of PKU. Early diagnosis is critical, as it allows for prompt initiation of treatment, which helps prevent severe neurological and developmental complications. Increased awareness among healthcare providers and the public, along with improved screening technologies, has contributed to the identification of more PKU cases, thereby expanding the addressable patient base and driving demand for treatments.
The market is witnessing a shift from traditional dietary management to more advanced pharmacological treatments, such as enzyme replacement therapies (e.g., pegvaliase) and small-molecule drugs (e.g., sapropterin dihydrochloride). These innovative therapies offer improved efficacy, convenience, and quality of life for patients, leading to higher adoption rates among physicians and patients alike. The introduction of injectable enzyme therapies has been particularly impactful, as they provide an alternative for patients who struggle with strict dietary restrictions or who do not respond to other treatments.
Expanded insurance benefits and government-funded reimbursement programs have significantly improved patient access to emerging PKU therapies. In the United States, policies such as the Newborn Screening Saves Lives Act and orphan drug incentives have facilitated the adoption of new treatments. These supportive reimbursement frameworks reduce the financial burden on patients and encourage the uptake of high-cost, advanced therapies.
Pharmaceutical and biotechnology companies are investing heavily in R&D to develop next-generation therapies, including gene therapies and novel enzyme formulations. This ongoing innovation is expected to broaden the range of available treatment options and further drive market growth. Strategic collaborations and partnerships between industry players and research institutions are also accelerating the development and commercialization of new therapies.
For instance, in March 2024, Eton Pharmaceuticals Inc., a company listed on the Nasdaq stock exchange under the ticker symbol “ETON”, is known for developing and bringing to market treatments for rare diseases. Eton announced that it has acquired the U.S. rights to a product called PKU GOLIKE from RELIEF THERAPEUTICS Holding SA (a Swiss company listed on the SIX Swiss Exchange as “RLF” and on OTC Markets in the U.S. as “RLFTF” and “RLFTY”).
Also, in January 2024, in the US, Jnana Therapeutics is a biotechnology company that uses advanced technology (next-generation chemoproteomics) to find new medicines for difficult-to-treat diseases. The company has shared positive, statistically significant interim results from an ongoing clinical study of JNT-517 in people with phenylketonuria (PKU). Thus, the above factors are consolidating the region's position as a dominant force in the global phenylketonuria treatment market.
Asia-Pacific phenylketonuria treatment market was valued at US$ 103.12 Million in 2024
The rising prevalence of PKU in Asia-Pacific is partly due to improved awareness, better diagnostic tools, and the gradual expansion of newborn screening programs. As more countries implement or enhance these screening initiatives, the detection of PKU at earlier stages is increasing, leading to earlier intervention and a growing patient population requiring treatment.
There is a growing shift from traditional dietary management to more advanced pharmacological treatments, such as enzyme replacement therapies (like pegvaliase) and small-molecule drugs (like sapropterin dihydrochloride). As awareness and regulatory approvals for these therapies increase, their adoption is expected to accelerate across the region.
Governments in the Asia-Pacific region are investing in healthcare infrastructure and rare disease management. Policies aimed at improving access to orphan drugs, such as fast-track approvals and incentives for local drug manufacturing, are helping to make advanced PKU treatments more accessible. For example, China’s inclusion of PKU in the Rare Disease Catalog and reforms aimed at pediatric PKU treatment coverage are notable developments.
Increased healthcare spending and greater patient and physician awareness of PKU are driving demand for effective therapies. As disposable incomes rise and health systems modernize, more patients can access specialized care and new treatment options.
Pharmaceutical companies are increasingly targeting Asia-Pacific through partnerships, collaborations, and market entry strategies. This is introducing new therapies and expanding access to innovative treatments, further stimulating market growth. Thus, the above factors are consolidating the region's position as a dominant force in the global phenylketonuria treatment market.
Global Phenylketonuria Treatment Market – Competitive Landscape (Major Players)
The major global players in the phenylketonuria treatment market include BioMarin Pharmaceutical Inc., Cycle Pharmaceuticals Limited, among others.
Global Phenylketonuria Treatment Market – Competitive Landscape (Emerging Players)
The emerging players in the phenylketonuria treatment market include PTC Therapeutics, Inc., NGGT Inc., Jnana Therapeutics Inc., and Sanofi, among others.
Global Phenylketonuria Treatment Market – Key Developments
In April 2025, Cycle Pharmaceuticals introduced Cycle Vita PKU, a new smartphone application designed to help patients manage Phenylketonuria (PKU) in their daily lives. PKU is an inherited metabolic disorder where the body cannot process phenylalanine (Phe), an amino acid in all protein-containing foods. Without lifelong dietary management, PKU can cause severe neurological and developmental issues.
In April 2025, PTC Therapeutics, Inc. announced that the Committee for Medicinal Products for Human Use (CHMP), which is part of the European Medicines Agency (EMA), had given a positive opinion on the marketing authorization application for Sephience (sepiapterin). This means the CHMP recommends that Sephience should be approved for use in Europe as a treatment for both children and adults with phenylketonuria (PKU).
In December 2024, Alltrna, a biotechnology company incubated by Flagship Pioneering that is leading the way in harnessing transfer RNA (tRNA) biology to create new therapies for disease, announced the unveiling of new preclinical findings. These results feature the company’s first tRNA-based drug candidate, AP003. In early studies, a single administration of AP003 restored robust, clinically relevant levels of protein production in mouse models engineered to mimic two genetic diseases: methylmalonic acidemia (MMA) and phenylketonuria (PKU).
In October 2024, PTC Therapeutics announced that the U.S. Food and Drug Administration (FDA) had accepted for filing the New Drug Application (NDA) for sepiapterin as a treatment for phenylketonuria (PKU) in both pediatric (children) and adult patients.
In July 2024, PTC Therapeutics, Inc. announced that it submitted a New Drug Application (NDA) for sepiapterin to the U.S. Food and Drug Administration (FDA). The NDA seeks approval for sepiapterin as a treatment for both children and adults with phenylketonuria (PKU), covering all ages and disease subtypes, meaning it is intended for use across the entire range of PKU patients, regardless of their specific genetic mutation or age group.
Global Phenylketonuria Treatment Market – Scope
Metrics | Details | |
CAGR | 8.4% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Type | Classic PKU, Mild PKU, Moderate PKU, Hyperphenylalaninemia, Others |
Drug Type | Sapropterin Dihydrochloride, Pegvaliase-pqpz, Others | |
Age Group | Adults, Pediatrics | |
Route of Administration | Oral, Parenteral | |
End-User | Hospitals, Specialty Clinics, Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global phenylketonuria treatment market report delivers a detailed analysis with 66 key tables, more than 68 visually impactful figures, and 173 pages of expert insights, providing a complete view of the market landscape.