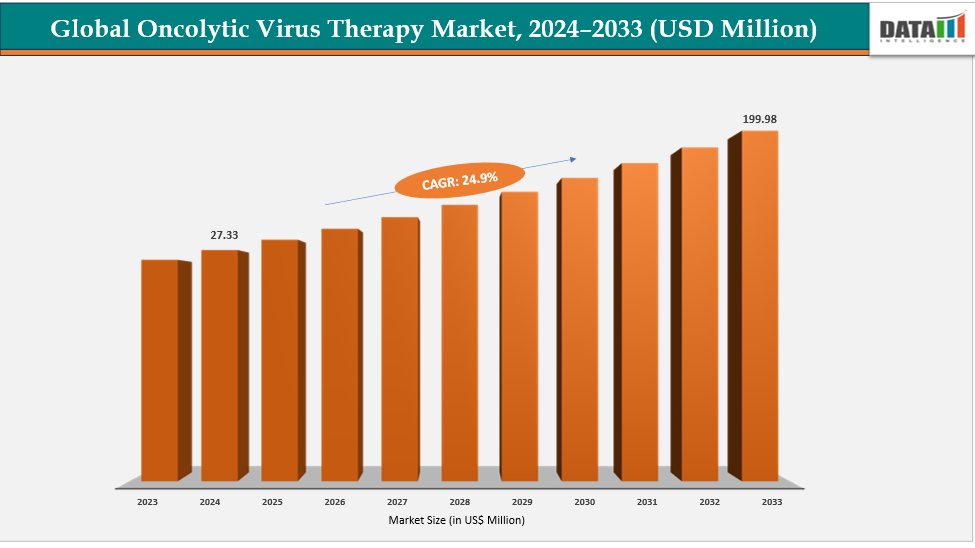
Oncolytic Virus Therapy Market Size & Industry Outlook
Advancements in viral engineering technology are reshaping the market for oncolytic virus therapies. Researchers are creating viruses that specifically target cancerous cells. These modified viruses are capable of delivering therapeutic genes to enhance the immune response. Recent innovations include equipping viruses with cytokines and checkpoint inhibitors. Delivery methods are advancing, enabling systemic administration instead of just intratumoral injections. Synergistic therapies alongside immunotherapies are demonstrating improved effectiveness.
AI and computational tools are being utilized to enhance viral design. Safety and tolerability profiles are being optimized through meticulous engineering. The number of clinical trial pipelines is rapidly increasing on a global scale. Regulatory bodies are progressively endorsing breakthrough designations. Production and CDMO capacities are expanding to keep pace with growing demand.
Key Highlights
- North America is dominating the global oncolytic virus therapy market with the largest revenue share of 48.5% in 2024.
- The Asia Pacific region is the fastest-growing region in the global oncolytic virus therapy market, with a CAGR of 7.7% in 2024.
- The Imlygic segment is dominating the oncolytic virus therapy market with a 60.3% share in 2024
- The herpes simplex virus type-1 is dominating the oncolytic virus therapy market with a 50.3% share in 2024
- Top companies in the oncolytic virus therapy market include Amgen, Inc., Shanghai Sunway Biotech Co., Ltd., and Daiichi Sankyo Co., Ltd. And emerging players are Oncolytics Biotech Inc., KaliVir Immunotherapeutics, Inc., TILT Biotherapeutics (TILT), Memgen, Inc., Genelux Corporation, CG Oncology, and Candel Therapeutics, Inc., among others.
Market Dynamics
Drivers: Strong research and development pipeline is accelerating the growth of the oncolytic virus therapy market
The robust research and development (R&D) pipeline is driving rapid expansion in the oncolytic virus therapy market. Various biotech and pharmaceutical firms are making substantial investments in creating innovative viral platforms that offer better tumor selectivity, safety, and immune stimulation. Many clinical trials are currently being conducted around the world, investigating the potential of combining oncolytic viruses with immune checkpoint inhibitors, CAR-T therapies, and targeted medications to improve treatment effectiveness.
For instance, in August 2025, Memgen, Inc. announced that it had dosed the first patient in its Phase 1 combination cohort evaluating intratumoral MEM-288 with standard-of-care docetaxel in patients with advanced or metastatic non–small cell lung cancer (NSCLC).
Moreover, in December 2024, Candel Therapeutics, Inc. announced positive results from its multicenter Phase 3 clinical trial, where CAN-2409 viral immunotherapy achieved the primary endpoint by significantly improving disease-free survival in patients with localized prostate cancer.
High manufacturing complexity and cost are hampering the growth of the oncolytic virus therapy market
The high complexity and cost of manufacturing pose major challenges to the expansion of the oncolytic virus therapy market. The production of these therapies necessitates sophisticated biomanufacturing facilities that comply with stringent biosafety standards (BSL-2/BSL-3) and Good Manufacturing Practices (GMP). This process involves intricate viral engineering, purification, and quality control measures to guarantee safety and effectiveness, leading to increased production costs.
Furthermore, ensuring viral stability requires specialized cold-chain logistics and storage solutions. The limited capacity for large-scale manufacturing and significant investment in research and development also contribute to elevated overall expenses. Consequently, oncolytic virus therapies remain costly, limiting availability, particularly in developing regions.
For more details on this report, see Request for Sample
Oncolytic Virus Therapy Market, Segment Analysis
The global oncolytic virus therapy market is segmented based on drug, virus type, indication, distribution channel, and region
By Drug: The Imlygic segment is dominating the oncolytic virus therapy market with a 60.3% share in 2024
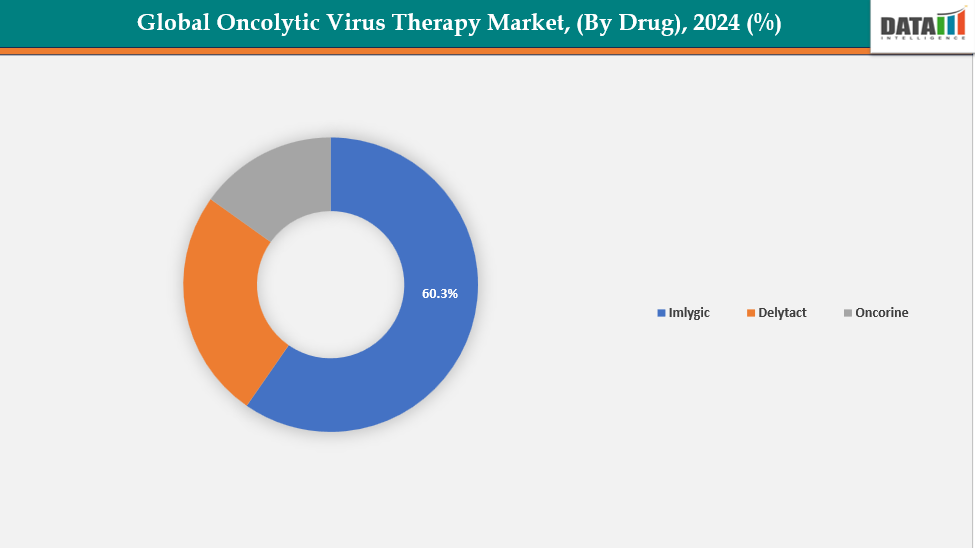
The Imlygic (Talimogene laherparepvec) segment leads the global market for oncolytic virus therapies, benefiting from its pioneering status as the first oncolytic virus therapy approved by the FDA for melanoma. Created by Amgen, Imlygic has built a robust commercial and clinical footprint since its approval in 2015. Its demonstrated effectiveness in treating advanced melanoma, along with a favorable safety profile and its combination with checkpoint inhibitors like pembrolizumab, have enhanced its widespread use.
In addition, the therapy's strong clinical evidence, widespread accessibility in key markets (U.S., Europe), and ongoing investigations for new cancer therapies enhance its leading status. Imlygic's high level of physician knowledge, well-established manufacturing capabilities, and current studies involving combination immunotherapies help maintain its prevailing market position.
By Virus Type: The herpes simplex virus type-1 is dominating the oncolytic virus therapy market with a 50.3% share in 2024
The Herpes Simplex Virus Type-1 (HSV-1) is leading the market for oncolytic virus therapy due to its robust genetic stability, extensive genome capacity, and capability to specifically target and destroy tumor cells while leaving healthy tissues unharmed. HSV-1 is easily modifiable to introduce immune-boosting genes, which improves anti-tumor immune responses. The approval of Imlygic (Talimogene laherparepvec), the first oncolytic therapy derived from HSV-1 for melanoma by the FDA, has confirmed its clinical viability and stimulated extensive research efforts.
Moreover, the continuous research and development and ongoing clinical trial make this segment dominant. For instance, in June 2025, ImmVira announced that the first patient had been dosed in its multi-regional Phase II clinical trial evaluating MVR-T3011, a genetically modified herpes simplex virus type-1 (HSV-1) oncolytic immunotherapy, for treating BCG-unresponsive high-risk non-muscle-invasive bladder cancer (NMIBC).
Oncolytic Virus Therapy Market, Geographical Analysis
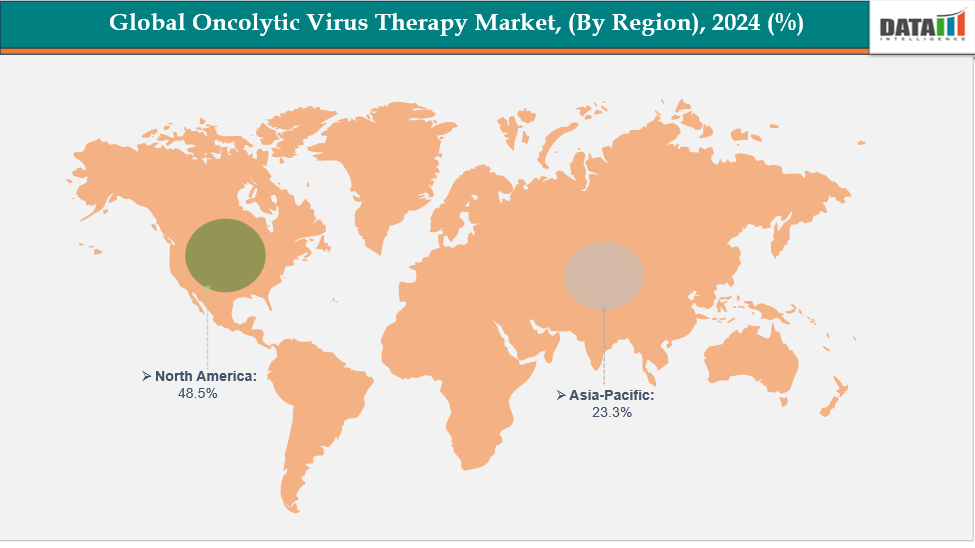
North America is dominating the global oncolytic virus therapy market with 48.5% in 2024
North America leads the oncolytic virus therapy market due to its well-established biotechnology sector, high incidence of cancer, sophisticated healthcare infrastructure, and early embrace of immuno-oncology therapies. Significant investments in research and development, supportive regulatory environments, the presence of prominent companies, and an increase in clinical trial activities all contribute to the region’s dominance in this market.
In the United States, the market for oncolytic virus therapies has expanded as a result of increasing cancer cases, FDA Fast Track approvals, strong investments in research and development, and the growth of clinical trials that are pushing forward next-generation viral immunotherapies for enhanced cancer treatment. For instance, in March 2024, ImmVira announced that the FDA had granted Fast Track designation to its oncolytic virus product MVR-T3011 IT (intratumoral injection) for treating recurrent or metastatic head and neck squamous cell carcinoma following prior chemotherapy and anti-PD-1/PD-L1 therapy.
Europe is the second region after North America, which is expected to dominate the global oncolytic virus therapy market with 34.5% in 2024
In Europe, the market for oncolytic virus therapy has expanded as a result of the increasing incidence of cancer, greater availability of advanced immunotherapies, and growth in clinical research efforts. Significant investments in research and development, swift regulatory approvals from the European Medicines Agency, and the early uptake of innovative therapies have improved patient access and sped up the advancement of next-generation oncolytic treatments.
Moreover, strategic collaborations and partnerships for novel molecule clinical trials accelerated oncolytic virus therapy market growth and treatment accessibility. For instance, in April 2024, Germany-based IDT Biologika collaborated with CanVirex to advance cancer treatment through their oncolytic virotherapy platform. The collaboration entered Phase I/IIa clinical trials to evaluate Mevacil, an oncolytic measles virus engineered to express interleukin-12 (IL-12), enhancing immune response and therapeutic efficacy in advanced-stage solid tumors.
The Asia-Pacific region is the fastest-growing region in the global oncolytic virus therapy market, with a CAGR of 7.7% in 2024
The market for oncolytic virus therapy in the Asia-Pacific region, which includes nations like China, Japan, South Korea, and India, experienced significant growth due to the increasing rates of cancer, heightened patient awareness, advancements in healthcare infrastructure, a rise in biologics manufacturing, favorable government policies, and ongoing collaborative research focused on improving targeted virotherapy and immuno-oncology therapies.
China’s Oncolytic Virus Therapy Market expanded rapidly, fueled by increasing cancer prevalence, supportive regulatory approvals, robust research and development, numerous ongoing clinical trials, and new product launches enhancing treatment availability and patient access. Owing to the factors like clinical trials, for instance, in March 2025, researchers at Sun Yat-sen University’s Third Affiliated Hospital achieved a milestone with VRT106, the first M1 hepatitis A virus-based oncolytic therapy, successfully initiating Phase I trials with the first patient dosed intravenously without adverse effects.
Competitive Landscape
Top companies in the oncolytic virus therapy market include Amgen, Inc., Shanghai Sunway Biotech Co., Ltd., and Daiichi Sankyo Co., Ltd. And emerging players are Oncolytics Biotech Inc., KaliVir Immunotherapeutics, Inc., TILT Biotherapeutics (TILT), Memgen, Inc., Genelux Corporation, CG Oncology, and Candel Therapeutics, Inc., among others.
Amgen, Inc.:Amgen, Inc., a global biotechnology leader, is advancing the oncolytic virus therapy market through innovative cancer treatments and strategic collaborations. Leveraging its expertise in biologics and immuno-oncology, Amgen focuses on research, development, and clinical trials to expand patient access and improve therapeutic outcomes in solid tumors.
Key Developments:
- In February 2025, UroGen Pharma expanded its oncology pipeline by acquiring the next-generation oncolytic virus ICVB‑1042 from IconOVir Bio and established multiple strategic research collaborations to leverage its RTGel technology, enhancing the clinical effectiveness and treatment outcomes of various immunotherapies.
- In September 2024, Virogin Biotech’s first-in-class oncolytic virus VG161, exclusively licensed to CNBG-Virogin for China, received Breakthrough Therapy Designation from the CDE, becoming the first oncolytic virus granted BTD for advanced hepatocellular carcinoma patients who had failed standard therapies.
Market Scope
| Metrics | Details | |
| CAGR | 24.9% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Mn) | |
| Segments Covered | By Drug | Imlygic, Delytact, Oncorine |
| By Virus Type | Herpes Simplex Virus type-1, Adenovirus type-5, Others | |
| By Indication | Head and Neck Cancer, Unresectable Melanoma, Malignant Glioma | |
| By Distribution Channel | Hospital Pharmacies, Retail Pharmacies | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global oncolytic virus therapy market report delivers a detailed analysis with 62 key tables, more than 53 visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more pharmaceutical-related reports, please click here