Global Non-Invasive Prenatal Testing (NIPT) Market: Industry Outlook
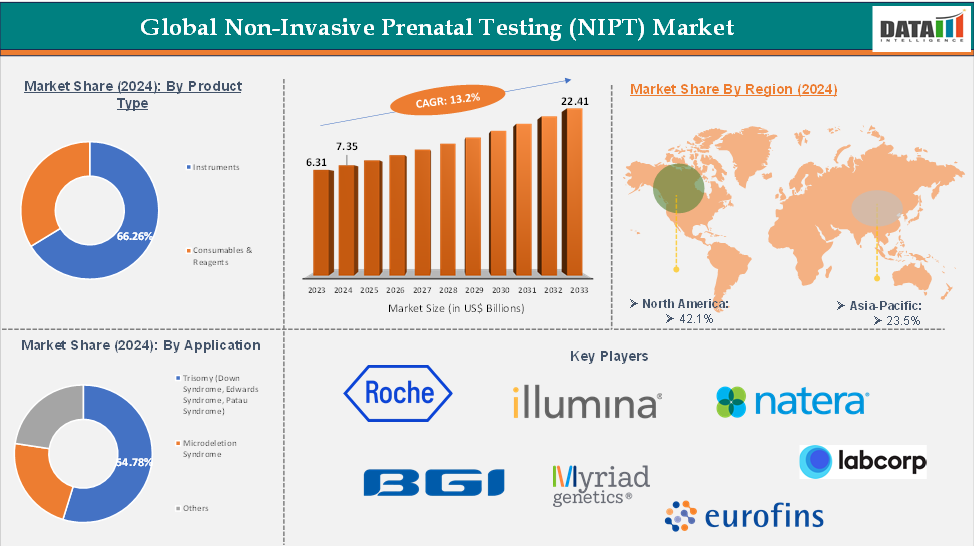
The global non-invasive prenatal testing (NIPT) market reached US$ 6.31 billion in 2023, with a rise of US$ 7.35 billion in 2024, and is expected to reach US$ 22.41 billion by 2033, growing at a CAGR of 13.2% during the forecast period 2025-2033.
The global non-invasive prenatal testing (NIPT) market is growing due to the increasing prevalence of chromosomal abnormalities, maternal age, and preference for safer, non-invasive alternatives. NIPT uses cell-free fetal DNA analysis to detect genetic conditions with high accuracy and minimal risk. Technological advancements in genomic sequencing, bioinformatics, and next-generation platforms enhance test efficiency. However, high testing costs and limited awareness in developing regions pose challenges. Expanding healthcare infrastructure, increasing awareness of early genetic screening, and broader adoption in emerging markets are expected to create significant growth opportunities in the NIPT market.
Executive Summary
Dynamics: Drivers & Restraints
Driver: Rising Prevalence of Chromosomal Abnormalities In Newborns
The global non-invasive prenatal testing (NIPT) market is expanding due to the increasing prevalence of chromosomal abnormalities in newborns, such as Down syndrome, Edwards syndrome, and Patau syndrome. This rise in incidence is driven by advancing maternal age and lifestyle-related risk factors, leading to a higher demand for early, accurate, and safe screening methods. Unlike invasive procedures, NIPT offers a non-invasive, highly sensitive, and low-risk option, making it a preferred choice for healthcare providers and patients. As awareness and the need for early detection intensify, the NIPT market continues to grow globally.
For instance, chromosomal abnormalities in newborns are a significant public health concern, with Down syndrome occurring in 29.7 per 10,000 births, 1 in every 336 births, and Edwards' syndrome and Patau's syndrome in 1 in 1,086 and 1 in 3,048 births, respectively, according to NHS England's NCARDRS data from 2021.
Restraint: High Cost of NIPT Procedures
The Global Non-Invasive Prenatal Testing (NIPT) Market faces significant challenges due to the high cost of NIPT procedures. In developed markets, the cost ranges between USD 800 and USD 2,000, while in emerging markets, it is around USD 300-500. This high cost creates an economic barrier for patients and healthcare systems, despite the test's accuracy and safety advantages. Despite its benefits, the high cost structure restricts access and adoption, slowing down the overall market penetration of NIPT worldwide.
For more details on this report, Request for Sample
Segmentation Analysis
The global non-invasive prenatal testing (NIPT) market is segmented by product type, test type, method, application, end user, and region.
Product Type:
The instruments from the product type segment the expected to have 24.66% of the non-invasive prenatal testing (NIPT) market share.
The instruments segment is gaining momentum due to advancements in next-generation sequencing platforms, automated sample preparation systems, and high-throughput genomic analyzers. The demand for precise chromosomal abnormality detection is driving the adoption of advanced sequencing instruments. Key players are investing in R&D to enhance instrument accuracy, reduce turnaround time, and integrate bioinformatics tools. The shift towards automation and scalable solutions is strengthening the role of instruments as a critical driver in the market's expansion.
For instance, in June 2025, BGI Genomics and Prom-Test Laboratories have localized the Non-Invasive Prenatal Testing (NIPT) project in Armenia, marking the first time such a project has been launched in the country, aiming to improve access to advanced prenatal diagnostics and health outcomes for expectant mothers.
Geographical Share Analysis
The North America global non-invasive prenatal testing (NIPT) market was valued at 42.1% market share in 2024
North America's strong healthcare infrastructure, high awareness of advanced prenatal screening, and early adoption of genomic technologies make it a key growth driver in the Global Non-Invasive Prenatal Testing (NIPT) Market. The region benefits from next-generation sequencing platforms, established diagnostic laboratories, and favorable reimbursement policies. The rising maternal age and chromosomal abnormalities increase demand for early screening solutions. North America's presence, ongoing clinical research, and regulatory approvals for new NIPT assays further strengthen its position.
For instance, in August 2025, Natera, a global leader in cell-free DNA testing and precision medicine, launched Fetal Focus, a noninvasive prenatal test for inherited conditions. The test addresses the unmet need for partner testing when a pregnant patient is identified as a carrier of a recessive single-gene condition. If the biological father is unavailable for testing, Fetal Focus can screen the fetus directly for the gene by analyzing a sample of the mother's blood. This is particularly useful when the biological father is unavailable for testing.
Competitive Landscape
The major players in the Non-Invasive Prenatal Testing (NIPT) market include Natera, Inc., Illumina, Inc., F. Hoffmann-La Roche Ltd, BGI Genomics Co., Ltd, Laboratory Corporation of America Holdings (LabCorp), Qiagen N.V., Eurofins Scientific, Agilent Technologies, Inc., Myriad Genetics, Inc., and among others.
Key Developments
In February 2025, Yourgene Health, part of the Novacyt group, launched IONA Care+, a comprehensive non-invasive prenatal screening service for genetic conditions in the UK. The service uses Yourgene's IONA Nx NIPT Workflow to deliver safe, fast, and accurate results, reducing the need for invasive tests and associated risks, while reducing stress and anxiety for expectant parents.
Report Scope
Metrics | Details | |
CAGR | 13.2% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segmentation | Product Type | Instruments, Consumables & Reagents |
Test Type | MaterniT21, Harmony, Panorama, Others | |
Method | Ultrasound Detection, Biochemical Screening Tests, Cell-Free DNA In Maternal Plasma | |
Application | Trisomy (Down Syndrome, Edwards Syndrome, Patau Syndrome), Microdeletion Syndrome, Others | |
End User | Diagnostic Laboratories, Hospitals, Clinics | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global non-invasive prenatal testing (NIPT) market report delivers a detailed analysis with 62 key tables, more than 61 visually impactful figures, and 195 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more clinical diagnostics-related reports, please click here