Myotonic Dystrophy Treatment Market is Segmented By Disease Type, Drug Class, Route of Administration, Distribution Channel, And By Region (North America, Latin America, Europe, Asia Pacific, Middle East And Africa) – Share, Size, Outlook, And Opportunity Analysis, 2024-2031
Market Overview
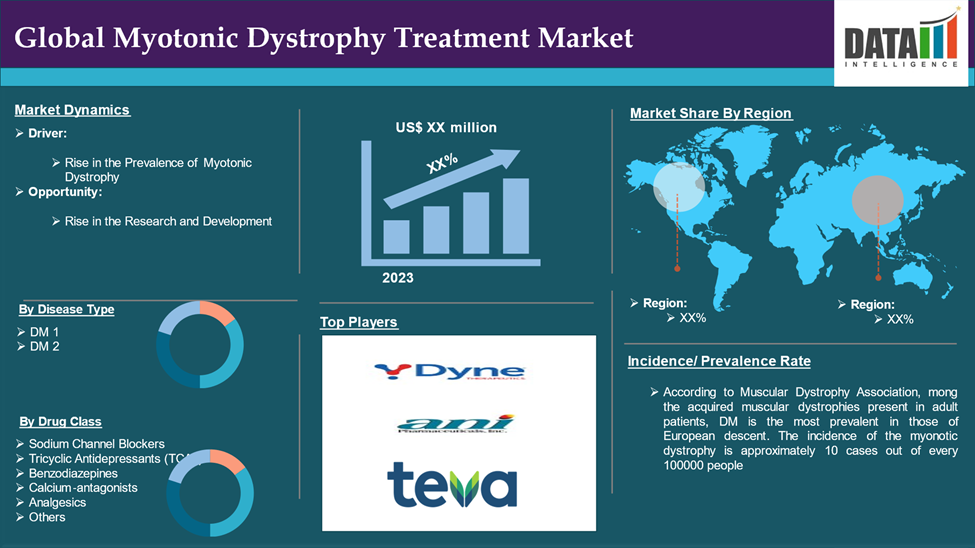
The global myotonic dystrophy treatment market reached US$ XX million in 2023 and is expected to reach US$ XX million by 2031, growing at a CAGR of XX% during the forecast period 2024-2031.
Myotonic dystrophy is an degenerative condition that is characterized with obligatory weakness in all people. Besides musculoskeletal weakness, cardiac conduction system defects and early onset of cataracts are prevalent as well. Myotonic Dystrophy exists in two forms, DM1 and DM2. Moreover, this activity focuses on the assessment and treatment aspects of myotonic dystrophy and outlines the importance of interprofessional teams in managing patients with this condition.
Executive Summary
Market Dynamics: Drivers & Restraints
Rise in the Prevalence of Myotonic Dystrophy
The increase in the number of people affected by myotonic dystrophy disease which helps to contribute towards the overall treatment market for myotonic dystrophy. With the diagnosis of this genetic disorder at muscle abuse that has multiple effects on a person over the years, there has been an urge for treatment to ease the suffering and make people live better. As such numbers of patients increase, pharmaceutical investors and researchers develop vision to develop new treatment approaches such as gene therapy, modern molecular drugs and drugs directed at alleviating the symptoms of the particular disease. This trend increases the extent of market growth as new treatment options that are effective and available are in high demand the world over.
For instance, according to Muscular Dystrophy Association, mong the acquired muscular dystrophies present in adult patients, DM is the most prevalent in those of European descent. The incidence of the myonotic dystrophy is approximately 10 cases out of every 100000 people. Within the ethnic groups that are not white, DM1 is relatively uncommon or non-existent. Research conducted in some parts of Europe indicates that the rate of occurrence of DM2 presents a similar picture as that of DM1.
Complications Associated with the Drugs
Myotonic dystrophy syndrome that affects skeletal muscles, the heart, the lungs, the digestive tract, etc., therefore predisposing the patient to more adverse effects and interactions with prescribed drugs. Standard drugs used for treating such conditions for example anticonvulsants can also bring about such side effects as sedation, dizziness, and stomach upset. They can also bring about liver damage or exacerbate heart failure, particularly in those with a history of heart conditions. There is also a risk of respiratory complications such as in cases when medications containing respiratory depressants do not suit a patient who has weakened respiratory muscles.
For more details on this report – Request for Sample
Segment Analysis
The global myotonic dystrophy treatment market is segmented based on disease type, drug class, route of administration, distribution channel, and region.
Drug Class:
Sodium channel blockers segment is expected to dominate the myotonic dystrophy treatment market share
The sodium channel blockers segment holds a major portion of the Myotonic Dystrophy Treatment market share and is expected to continue to hold a significant portion of the myotonic dystrophy treatment market share during the forecast period.
The management of myotonic dystrophy is significantly dependent on the use of sodium channel blockers, which target the muscle stiffness and lingering contraction (myotonia) observed with this condition. Such drugs will block sodium channels in muscle cells, preventing excessive action potential firing and intruding muscle contractions. Medications such as mexiletine, which is a sodium channel blocker, are the most favored due to the myotonia reduction they attain and the attendant gain in terms of mobility and living standards of patients.
The use of sodium channel blockers is intended to alleviate these symptoms while sparing muscle function in general, by stabilizing the membrane of the muscle and curbing the excess excitation. Nevertheless, because of some side effects such as cardiovascular and neurological ones, dosing and monitoring must be done properly. This targeted approach highlights the importance of sodium channel blockers in the treatment of myotonic dystrophy since they play a role in alleviating one of the main features of the disease.
For instance, in January 2024, New research from the University of Rochester Medical Center identifies the biological mechanism behind muscle dysfunction in myotonic dystrophy type 1 (DM1) and demonstrates that calcium channel blockers can reverse these symptoms in animal models of the disease, suggesting these drugs for the treatment for DM1.
Tricyclic Antidepressants (TCAs) segment is the fastest-growing segment in the myotonic dystrophy treatment market share
The tricyclic antidepressants (TCAs) segment is the fastest-growing segment in the myotonic dystrophy treatment market share and is expected to hold the market share over the forecast period.
Tricyclic antidepressants are used in the treatment of myotonic dystrophy in some instances due to their effect on the neuropathic pain, depression, and other mood disorders rather than specifically treating myotonia itself. As a multisystem disorder affecting the nervous system, myotonic dystrophy often leads to depressive and anxiety symptoms as well as chronic pain which are detrimental to health and quality of life in general.
Tricyclic antidepressants (TCAs) such as amitriptyline and nortriptyline not only helps patients improve their moods but also helps treat nerve pain promoting slight sedation that may be effective in dealing with muscle tension. At the same time, there is a danger of adverse effects from the TCAs such as excessive sleepiness, dry mouth and possible effects on the heart especially so in patients with myotonic dystrophy where the heart is compromised.
Disease Type:
DM 1 segment is expected to dominate the myotonic dystrophy treatment market share
The DM 1 segment holds a major portion of the myotonic dystrophy treatment market share and is expected to continue to hold a significant portion of the myotonic dystrophy treatment market share during the forecast period.
Myotonic dystrophy type 1 is a genetic neuromuscular disorder. It is caused by the expansion of the cytosine–thymine–guanine (CTG) trinucleotide repeat in the dystrophia myotonica protein kinase (DMPK) gene. Known for myotonia and facial and distal dominant weakness, it is now a multisystemic disease involving cataract, diabetes, arrhythmia, and central nervous system diseases. Patients with neuromuscular diseases have a higher risk of postoperative complications due to underlying muscle weakness, scoliosis, and cardiac abnormalities.
For instance, in October 2024, Researchers from the Human Translational Genomics Group of Instituto de Investigación Sanitaria de INCLIVA-Universitat de València (UV) have discovered a promising therapy using antimiRs to treat myotonic dystrophy type 1 (DM1). The study, led by Dr. Rubén Artero, was published in Science Advances. The research was conducted in collaboration with Arthex Biotech, based on findings from INCLIVA, UV, the Badalona Neuromuscular Research Group, IGTP, Biogipuzkoa Health Research Institute, and the Health Research Institute Hospital La Fe.
Moreover, in December 2023, PepGen Inc., a biotechnology company, has dosed the first patient in its Phase 1 clinical trial, FREEDOM-DM1, evaluating PGN-EDODM1 for the treatment of DM1, aiming to revolutionize the treatment of severe neuromuscular and neurological diseases.
DM 2 segment is the fastest-growing segment in the myotonic dystrophy treatment market share
The DM 2 segment is the fastest-growing segment in the myotonic dystrophy treatment market share and is expected to hold the market share over the forecast period.
Myotonic dystrophy type 2 (DM2) is characterized by myotonia and muscle dysfunction (proximal and axial weakness, myalgia, and stiffness), occasionally associated with posterior subcapsular cataracts, cardiac conduction abnormalities, insulin-resistant diabetes mellitus and other endocrine disorders. While myotonia (involuntary contraction of a muscle or muscle group that is undergoing tension but not abdicating) has been often reported within the first ten years of life, it more often occurs from the third or fourth decades of life. This is usually with variable or recurring muscle pain, which can at times be severe, associated with proximal axial weakness of the neck and hip flexors, respectively.
Thereafter, weakness is seen in the elbow extensors and finger flexors, while weakness of facial musculature and the ankle dorsiflexors is rarer. Most cases of myotonia do not manifest significant signs or symptoms. There is also a group of individuals who are noted with calf hypertrophy and brisk reflexes.
Geographical Analysis
North America is expected to hold a significant position in the myotonic dystrophy treatment market share
North America holds a substantial position in the myotonic dystrophy treatment market and is expected to hold most of the market share due to the advanced healthcare infrastructure, strong research, and increasing awareness of rare diseases. Established pharmaceutical companies and academic institutions are working on innovative treatments, including gene therapies and targeted molecular drugs. The aging population and government support for rare disease research and favorable reimbursement policies further drive market growth, making therapies more accessible.
For instance, in September 2023, Dyne Therapeutics, Inc., a company developing therapeutics for muscle diseases, aimed at patients with genetically-oriented conditions, is glad to announced submission of application for orphan drug designation for treatment of myotonic dystrophy type 1 (DM1) with DYNE-101 to the US FDA, and received approval for it. Currently, DYNE-101 is undergoing evaluation in the Phase 1/2 global ACHIEVE clinical trial with initial data on safety, tolerability and splicing from the multiple ascending dose, placebo-controlled portion of the trial expected in late 2023.
Europe is growing at the fastest pace in the myotonic dystrophy treatment market
Europe holds the fastest pace in the myotonic dystrophy treatment market and is expected to hold most of the market share is driven by robust healthcare systems, specialized clinics, and personalized medicine. European countries are focusing on rare and genetic disorders, leading to better management. The high prevalence of the disease, ongoing clinical trials, and advancements in therapeutic options boost the market. The European Medicines Agency's approval of new treatments and support of rare disease organizations contribute to increased market awareness and treatment accessibility.
For instance, in October 2024, ARTHEx Biotech S.L.a clinical-stage biotechnology company focused on developing innovative medicines through the modulation of gene expression, announced that the first participant has been dosed in the Randomized Placebo Controlled Double Blind Phase I-IIa ArthemiR Trial of ATX-01 for Myotonic Dystrophy Type 1 (DM1). DM1 is an extremely debilitating and exceptional neuromuscular disorder that primarily affects patients through muscle weakness, among other complications that shorten their lifespan. Currently there are no treatments available that modify the course of DM1.
Competitive Landscape
The major global players in the myotonic dystrophy treatment market include Dyne Therapeutics, Teva Pharmaceuticals, Ani Pharmaceuticals, AA Pharma, Alkem Laboratories Ltd among others.
Emerging Players
The emerging players in the myotonic dystrophy treatment market include Vertex Pharmaceuticals, Avidity Biosciences, PepGen, and among others.
| Metrics | Details | |
| CAGR | XX% | |
| Market Size Available for Years | 2022-2031 | |
| Estimation Forecast Period | 2024-2031 | |
| Revenue Units | Value (US$ Mn) | |
| Segments Covered | Disease Type | DM1, DM2 |
| Drug Class | Sodium Channel Blockers, Tricyclic Antidepressants (TCAs), Benzodiazepines, Calcium‐antagonists, Analgesics, Others | |
| Route of Administration | Oral, Injectable, Topical | |
| Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and Middle East & Africa | |
Key Developments
- In May 2024, AMO Pharma made a revelation that phase 3 clinical trials although aimed at assessing the efficacy and safety of AMO-02, an experimental drug for the treatment of type And Myotonic Dystrophy (DM1) in adult patients diagnosed with adult onset DM1 will also be done. Evidence from that study together with the results of the phase 2/3 REACH-CDM trial (NCT05004129) are anticipated to be used as support in the registration submission of AMO-02 to the treatment of DM1.
- In January 2024, Dyne Therapeutics, Inc., a clinical-stage company working on debilitating muscle diseases, and whose goal is to develop cutting-edge life-changing treatments for patients suffering from genetically caused diseases, announced positive preliminary clinical results from ACHIEVE study of DYNE-101 for myotonic dystrophy type 1 (DM1) and DELIVER study of DYNE-251 for patients with Duchenne muscular dystrophy (DMD).
Why Purchase the Report?
- To visualize the global myotonic dystrophy treatment market segmentation based on disease type, drug class, route of administration, distribution channel and region and understand key commercial assets and players.
- Identify commercial opportunities by analyzing trends and co-development.
- Excel data sheet with numerous data points of the myotonic dystrophy treatment market with all segments.
- PDF report consists of a comprehensive analysis after exhaustive qualitative interviews and an in-depth study.
- Product mapping is available in excel consisting of key products of all the major players.
The global myotonic dystrophy treatment market report would provide approximately 70 tables, 65 figures, and 184 pages.
Target Audience 2023
- Manufacturers/ Buyers
- Industry Investors/Investment Bankers
- Research Professionals
- Emerging Companies