Mass Spectrometry Market – Industry Trends & Outlook
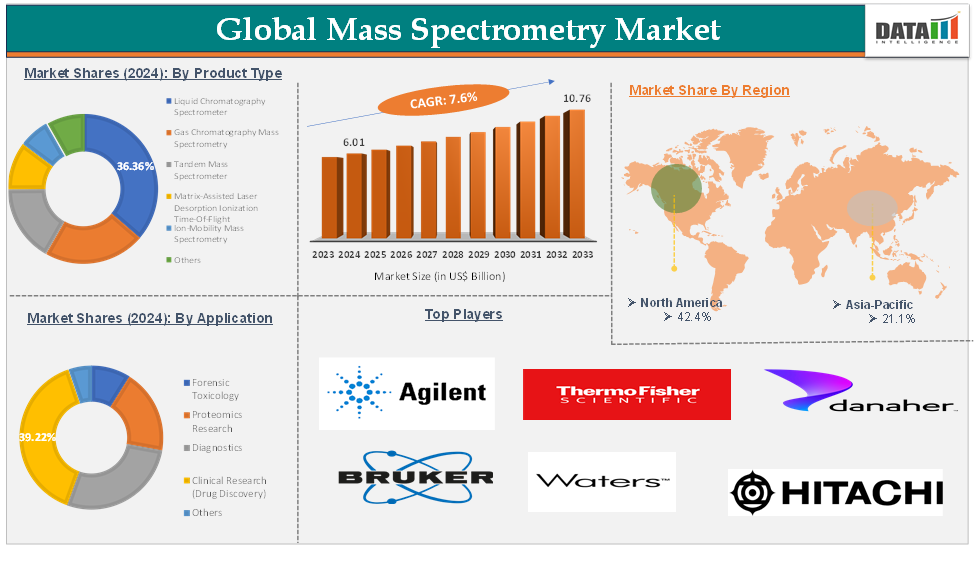
Mass Spectrometry Market reached US$ 6.01 Billion in 2024 and is expected to reach US$ 10.76 Billion by 2033, growing at a CAGR of 7.6 % during the forecast period of 2025-2033.
Mass spectrometry (MS) is a powerful analytical technique used to identify, quantify, and analyze chemical compounds based on their mass-to-charge ratio. The technology has become indispensable in a range of scientific and industrial fields due to its precision and sensitivity. Applications are expanding across sectors, with particular growth in forensic toxicology, diagnostics, clinical research, proteomics research, and others.
The global mass spectrometry market is witnessing robust growth, fueled by increasing demand in pharmaceutical development, clinical diagnostics, environmental testing, and food safety applications. With continued innovation in instrumentation and growing adoption in precision medicine and omics research, the market is projected to maintain strong momentum through 2033.
Executive Summary
For more details on this report, Request for Sample
Mass Spectrometry Market Dynamics: Drivers
New product launches and technological advancements
Rising novel product launches and technological advancements in the field of mass spectrometry are also expected to drive the market over the forecast period. Technological advancements often lead to the development of mass spectrometers with improved performance metrics such as sensitivity, resolution, and speed of analysis. This allows researchers to detect and quantify analytes at lower concentrations and with greater accuracy, making mass spectrometry an even more valuable tool across various industries.
For instance, in January 2024, Nova launched the second-generation Nova Metrion system for in-line secondary ion mass spectrometry (SIMS). The new Metrion incorporates key improvements, including increased sensitivity and enhanced charge compensation.
The Metrion system introduces a unique in-line composition profiling capability for key process control measurements, including contamination, residues, and diffusion, which cannot be obtained by other measurement techniques. This latest version also delivers faster time to results, improved dopant uniformity control, and better depth resolution.
Mass Spectrometry Market Dynamics: Restraints
The premium product pricing
The premium product pricing is expected to hamper the mass spectrometry market. High product pricing can deter potential customers, especially those with budget constraints such as academic institutions, smaller research laboratories and start-up companies. These organizations may opt for lower-cost alternatives or delay purchasing decisions, leading to slower adoption rates of mass spectrometry technology.
The high price of mass spectrometry instruments can limit market penetration, particularly in emerging markets and developing regions where healthcare and research budgets are limited. This could result in missed opportunities for manufacturers to expand their customer base and increase market share.
For instance, according to Harvard University, for mass spectrometry, the HPLC or GC methods development charges are $74.29 per hour for Harvard, $127.03 per hour for Harvard Affiliates, $141.14 per hour for non-Harvard academic or non-profit organizations and $185.72 for Corporate researchers. These premium product pricing limits the adoption of mass spectrometry techniques.
Manufacturers offering high-priced mass spectrometry instruments may face competitive disadvantages compared to rivals offering more affordable alternatives. Lower-cost competitors could gain market share by targeting price-sensitive customers or offering comparable performance at a lower price point, thereby eroding the market share of premium-priced products. Thus, the above factors could be limiting the global Mass Spectrometry market's potential growth.
Mass Spectrometry Market - Segment Analysis
The global mass spectrometry market is segmented based on product type, application, and region.
Product Type:
The liquid chromatography spectrometer product type segment is expected to hold 36.36 % of the global mass spectrometry market in 2024
Liquid chromatography mass spectrometry is an analytical technique that combines chromatographic separation of target compounds with mass-based detection. Its sensitivity, selectivity, and accuracy make it ideal for detecting nanomolar or picomolar quantities of various analytes, including drugs, food metabolites, biomarkers, pesticides, food contaminants, ecosystem stability markers, and natural product extracts.
An LC-MS system uses liquid chromatography to separate complex mixtures into components, which are then transferred to a mass spectrometer. The spectrometer ionizes molecules, separates ions based on their mass-to-charge ratio, and detects them to determine their identity and abundance.
Unlike gas chromatography mass spectrometry, liquid chromatography mass spectrometry using electrospray ionization (ESI) is capable of detecting nonvolatile, polar, and thermally labile compounds and provides a means of detecting a broad menu of drugs and metabolites without the need for lengthy sample preparations. The sample preparation time is usually significantly reduced for LC-MS methods compared to GC-MS methods since hydrolysis and derivatization are not required.
In addition, it has several other advantages, such as a more extensive linear dynamic range (typically), improved lower detection limits, higher accuracy, and precision because of the option to apply internal standards; the capability to quantify multiple analytes simultaneously; and the fact that it does not necessarily require immunological reagents.
For instance, in February 2024, Roche Diagnostics (Basel, Switzerland) developed a fully automated, standardized liquid chromatography-mass spectrometry (LC-MS) solution that will seamlessly integrate into existing clinical chemistry and immunochemistry testing as part of its cobas Pro integrated solutions, as well as laboratory automation and IT systems. These factors have solidified the segment's position in the global mass spectrometry market.
Global Mass Spectrometry Market – Geographical Analysis
North America is expected to hold 42.4% of the global mass spectrometry market in 2024
The North America mass spectrometry market is primarily driven by the robust demand for advanced analytical tools in the pharmaceutical and biotechnology industries, where mass spectrometry is crucial for drug development, quality control, and chemical composition analysis.
The region's well-established pharmaceutical sector, combined with significant investments in research and development, underpins this growth. Additionally, stringent environmental regulations and food safety standards have accelerated the adoption of mass spectrometry in environmental monitoring and food quality testing.
The increasing use of mass spectrometry in clinical diagnostics, particularly for personalized medicine and disease biomarker discovery, further contributes to market expansion. Other key drivers include strong government and private sector funding for R&D, the presence of leading market players and research institutions, and a growing focus on innovation and technological advancements such as hybrid and portable mass spectrometers. These factors collectively ensure North America's continued dominance in the global mass spectrometry market.
For instance, in June 2024, at the American Society for Mass Spectrometry (ASMS) conference held earlier this week in Anaheim, California, an instrument manufacturer unveiled its latest mass spectrometer, the Xevo Multi Reflecting Time-of-Flight (MRT). This new instrument builds upon the foundation of the SELECT SERIES MRT. Thus, the above factors are consolidating the region's position as a dominant force in the global Mass Spectrometry market.
Asia Pacific is expected to hold 21.1% of the global mass spectrometry market in 2024
The Asia-Pacific mass spectrometry market is experiencing robust growth, driven by several key factors. Increasing adoption of advanced analytical technologies in the pharmaceutical and biotechnology industries is a primary driver, as mass spectrometry is essential for drug discovery, development, and quality control.
The region's expanding pharmaceutical sector, particularly in countries like China, Japan, and India, is fueling demand for high-throughput and precise analytical instruments. Government initiatives and supportive funding for research and development, especially in healthcare, life sciences, and environmental sectors, are further accelerating market growth.
Additionally, there is a rising emphasis on food safety and environmental monitoring, with regulatory bodies enforcing stricter standards that necessitate the use of mass spectrometry for contaminant detection and quality assurance. The technology's growing application in clinical diagnostics, such as proteomics, metabolomics, and personalized medicine, also plays a significant role, as it enables early disease detection and tailored therapeutic approaches.
Technological advancements, including the development of hybrid and portable mass spectrometers, are expanding the market’s reach into new applications and improving accessibility in emerging economies. Collectively, these drivers are positioning Asia-Pacific as one of the fastest-growing regions in the global mass spectrometry market.
For instance, in January 2025, Medical & Biological Laboratories Co., Ltd. launched two new MALDI-TOF mass spectrometers, the Autof ms 1600 and ms 2600, which offer fast and reliable identification of microorganisms. These devices are widely used in healthcare, research, and food safety, and will be featured at a major clinical microbiology conference in Japan. Their proven reliability is demonstrated by the sale of over 800 units worldwide. Thus, the above factors are consolidating the region's position as a dominant force in the global Mass Spectrometry market.
Mass Spectrometry Market – Major Players
The major global players in the mass spectrometry market include Agilent Technologies, Bruker Corporation, Danaher Corporation, Hitachi Ltd, JEOL Ltd, LECO Corporation, MKS Instruments, PerkinElmer Inc., Shimadzu Corporation, Thermo Fisher Scientific Ltd, and Waters Corporation, among others.
Key Developments
In December 2024, Roche announced the launch of its cobas Mass Spec solution following receipt of Conformité Européenne (CE) mark approval. The launch includes the cobas i 601 analyzer and, as highlighted in the company’s press release, the first Ionify reagent pack featuring four assays for steroid hormones.
In June 2024, Agilent Technologies Inc. introduced two new mass spectrometry instruments designed to improve precision and sensitivity in scientific measurements. This announcement was made at the 72nd ASMS Conference on Mass Spectrometry and Allied Topics.
In August 2024, Thermo Fisher Scientific launched the EXENT Solution after receiving IVDR certification. The EXENT solution is a fully integrated and automated mass spectrometry system designed to transform diagnosis and assessment for patients with monoclonal gammopathies, including multiple myeloma.
In April 2024, Biognosys, a global leader in mass spectrometry-based proteomics, and Alamar Biosciences, Inc., a company powering precision proteomics to enable the earliest detection of disease, announced a strategic partnership aimed at advancing scientific discovery in the field of biofluid proteomics biomarkers. In February 2024, SCIEX launched the Echo MS+ system. The system couples proprietary Acoustic Ejection Mass Spectrometry technology and Open Port Interface (OPI) sampling with the capabilities of either the SCIEX ZenoTOF 7600 or Triple Quad 6500+ system to deliver precise qualitative and quantitative results through an expanded panel of robust high-throughput screening workflows.
In February 2024, SCIEX launched the Echo MS+ system. The system couples proprietary Acoustic Ejection Mass Spectrometry technology and Open Port Interface (OPI) sampling with the capabilities of either the SCIEX ZenoTOF 7600 or Triple Quad 6500+ system to deliver precise qualitative and quantitative results through an expanded panel of robust high-throughput screening workflows.
In January 2024, Shimadzu Scientific Instruments introduced its next-generation gas chromatograph mass spectrometer – the GC-MS-QP2050. Powered by advanced automated technology in a compact footprint, the GCMS-QP2050 features exceptional reliability, sensitivity, stability, and speed in an easy-to-use system.
In January 2024, Shimadzu Scientific Instruments introduced the ICPMS-2040/2050 Series of Inductively Coupled Plasma Mass Spectrometers that feature a proprietary advanced mini-torch system, redesigned collision/reaction cell, and a high-performance quadrupole mass filter with enhanced resolution.
Market Scope
Metrics | Details | |
CAGR | 7.6% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Product Type | Liquid Chromatography Spectrometer, Gas Chromatography Mass Spectrometry, Tandem Mass Spectrometer, Matrix-Assisted Laser Desorption Ionization Time-Of-Flight, Ion-Mobility Mass Spectrometry, Others |
Application | Forensic Toxicology, Proteomics Research, Diagnostics, Clinical Research (Drug Discovery), Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |