Janus Kinase (JAK) Inhibitors Market Overview
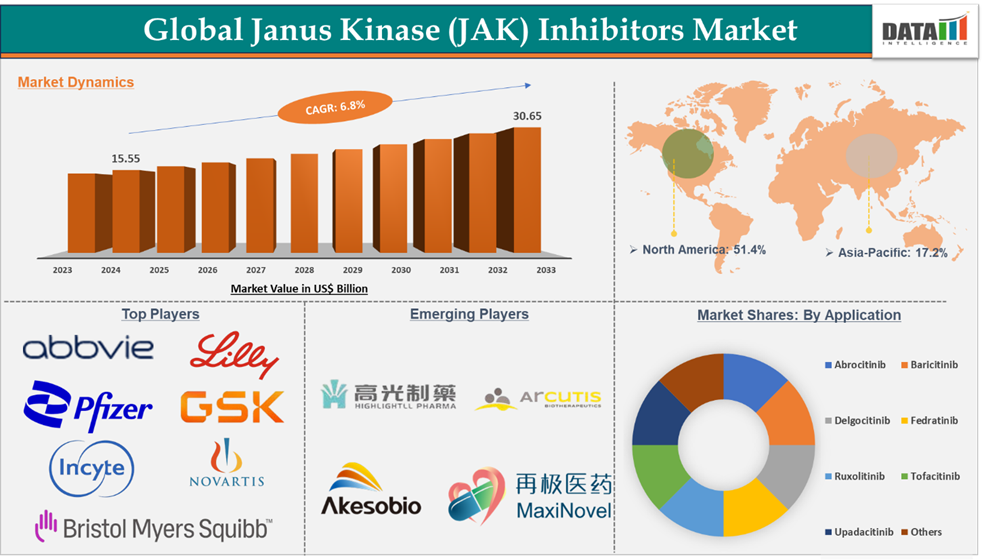
Janus Kinase (JAK) Inhibitors Market reached US$ 15.56 billion in 2024 and is expected to reach US$ 30.65 billion by 2033, growing at a CAGR of 6.8% during the forecast period 2025-2033, according to DataM Intelligence report.
Janus kinase is a group of proteins that are involved in the cytokine signaling pathway. These are known to play a crucial role in several autoimmune, allergic, and inflammatory conditions. There are notably four Janus kinase (JAK) proteins, named JAK1, JAK2, JAK3, and TYK2. These interact with seven signal transducers and activators of transcription (STAT) transcription factors to mediate intracellular signal transduction downstream of cytokine receptors.
Janus Kinase (JAK) inhibitors are drugs indicated in various conditions and are specifically known to inhibit the Janus kinase pathway. JAK1 is a key kinase involved in pruritic dermatitis, allergic rhinitis, asthma, and inflammatory bowel disease. Small-molecule inhibitors targeting JAK1 have shown efficacy in treating these diseases. Small molecules with JAK1 and JAK2 inhibitory activity have also provided therapeutic benefits in rheumatoid arthritis, psoriasis, and pruritus. JAK3 selective inhibitors and JAK1/TYK2 dual inhibitors have potential therapeutic options in inflammatory and autoimmune diseases.
The market for Janus Kinase (JAK) inhibitors is expected to see lucrative growth in the future driven by factors such as increasing efforts by manufacturers to discover, develop, and commercialize novel drugs, rising prevalence of targeted diseases, and increasing demand for novel therapies.
Executive Summary
For more details on this report – Request for Sample
Janus Kinase (JAK) Inhibitors Market Dynamics: Drivers & Restraints
The rising product development activities and market approvals are driving the market growth
The market for janus kinase (JAK) inhibitors is expected to be significantly driven by rising product development activities and subsequent market approvals. Established manufacturers and emerging players are actively involved in developing novel JAK inhibitors and also extensively conducting clinical trials to explore new indications for the approved JAK inhibitors.
For instance, in June 2024, Sun Pharmaceutical Industries Limited announced that the U.S. Food and Drug Administration (FDA) approved LEQSELVI (deuruxolitinib) 8 mg tablets for the treatment of adults with severe alopecia areata.
For instance, in September 2023, the U.S. Food and Drug Administration (FDA) approved Ojjaara, a JAK inhibitor developed by GSK Plc, for the treatment of myelofibrosis patients. Omjjaara was then approved in the EU and UK in January 2024 for the same indication.
Moreover, in April 2023, the European Commission (EC) approved Rinvoq, a JAK inhibitor developed by AbbVie for the treatment of adults with moderately to severely active Crohn’s disease who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) inhibitors. Subsequently, in May 2023, the U.S. FDA approved Rinvoq for the same indication.
This increasing product launch is expected to create significant traction in various therapeutic areas with high unmet needs and propel the overall market growth.
The side effects associated with Janus kinase (JAK) inhibitors may restrain the market growth.
Janus kinase (JAK) inhibitors, although revolutionizing the treatment scenario for several immunity-related conditions, are associated with certain risks and side effects. The serious side effects of JAK inhibitors include increased risk for infections, cardiovascular events (stroke, embolism, thrombosis, etc), and neoplasms.
A study published in The Nature Journal Scientific Reports in May 2022 has evaluated the adverse events associated with the first three approved JAK inhibitors: ruxolitinib, tofacitinib, and baricitinib. The study evaluated 126,815 reports from the WHO pharmacovigilance database, and the most common adverse events reported are
Increased incidence of infectious disease, especially viral infections such as herpes, influenza, fungal infections, and mycobacterial infectious disorders.
Musculoskeletal and connective tissue disorders
Cardiovascular events such as thrombosis and embolism
Cancers, especially malignant skin neoplasms.
The adverse effects are life-threatening and can impair the patient's outcome. This can lead to a decreased adoption rate and may impair market growth.
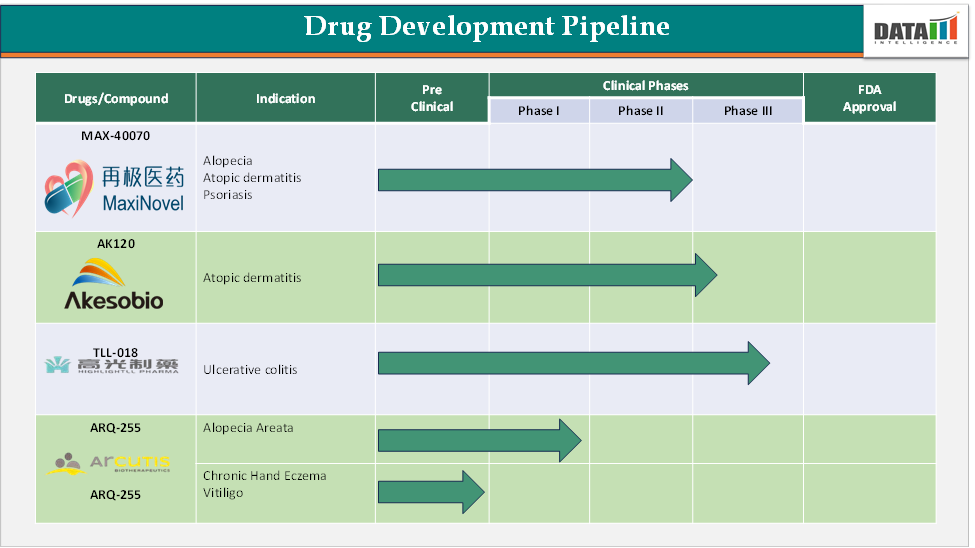
Janus Kinase (JAK) Inhibitors Market Pipeline Analysis
Janus Kinase (JAK) Inhibitors Market Segment Analysis
The global janus kinase (JAK) inhibitors market is segmented based on product, mechanism, indication, and region.
Upadacitinib in the product segment is growing with the highest CAGR in the product segment of Janus kinase (JAK) inhibitors market.
Upadacitinib, sold under the brand name Rinvoq, is a JAK inhibitor approved for the treatment of multiple indications, including eczema, psoriatic arthritis, non-radiographic axial spondyloarthritis, Crohn’s disease, rheumatoid arthritis, ankylosing spondylitis, and ulcerative colitis etc. Among children, the drug is approved for active polyarticular juvenile idiopathic arthritis and active psoriatic arthritis. Rinovoq is manufactured by AbbVie and is available as a once-daily pill for all the above indications.
The drug is approved in major markets such as North America, the European Union, and Japan for different indications.
Major Market | Approved Indication |
U.S | Rheumatoid arthritis, Psoriatic arthritis, Ankylosing spondylitis, Atopic dermatitis, Axial spondyloarthropathy, Ulcerative colitis, Crohn’s disease) |
European Union | Rheumatoid arthritis, Psoriatic arthritis, Ankylosing spondylitis, Atopic dermatitis, Axial spondyloarthropathy, Ulcerative colitis, Crohn’s disease) |
Canada | Psoriatic arthritis, Atopic dermatitis, Rheumatoid arthritis |
Japan | Rheumatoid arthritis, Psoriatic arthritis, Atopic dermatitis |
Rinvoq sales are skyrocketing each year, mainly due to expanding applications and geographic reach. AbbVie has generated US$ 47 million in the launch year 2019 and has generated US$ 5.97 billion in 2024, which is a growth of 163.5% in 5 years.
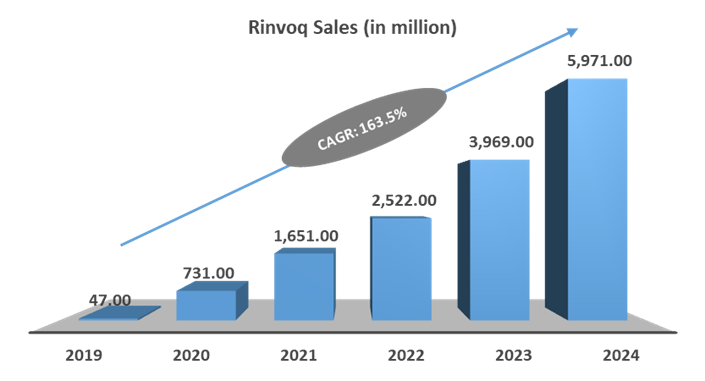
Moreover, AbbVie is expanding the clinical applications of Rinvoq by exploring its potential in newer indications. Rinvoq is being explored in phase 3 clinical trials for Alopecia Areata, Hidradenitis Suppurativa, Systemic Lupus Erythematosus (SLE), Takayasu Arteritis, and Vitiligo. This can further boost Rinvoq sales in the forecasted period.
Janus Kinase (JAK) Inhibitors Market Geographical Analysis
North America dominated the janus kinase (JAK) inhibitors market with the highest share of 51.4% in 2024
North America currently holds a significant market share in the Janus Kinase (JAK) Inhibitors market due to various factors, including higher demand for novel therapies, major revenue generated by market players, and higher prevalence of autoimmune disorders. Any emerging drug is expected to first get launched in the U.S. market due to higher demand, and all the Janus Kinase (JAK) Inhibitors in the current market were first launched in the U.S., providing first access to the patients in the country. Due to higher per capita income and spending on healthcare, the Janus Kinase (JAK) Inhibitors are sold at higher prices in the North America market as compared to other nations. Due to this, manufacturers generate a majority of their revenue from the region.
For instance, below are the top 3 selling Janus kinase (JAK) inhibitors and their revenue share by region.
Sr. No | Name of the drug/Brand | Manufacturer | Total Sales (2024) | U.S. Share |
1 | JAKAFI/JAKAVI | Incyte. & Novartis AG | 4,628.11 | 60.33% |
2 | Rinvoq | AbbVie Inc. | 5,971.00 | 71.33% |
3 | Xeljanz | Pfizer Inc. | 1,168.00 | 58.22% |
Janus Kinase (JAK) Inhibitors Market Major Players
The major global players in the Janus Kinase (JAK) Inhibitors market are Eli Lilly and Company., Bristol Myers Squibb company., GSK Plc., Pfizer Inc., Incyte., AbbVie Inc., Astellas Pharma Inc., LEO Pharma A/S, Torii Pharmaceutical Co., Ltd, and Sobi, Inc., among others.
Emerging Players
The emerging players in the Janus Kinase (JAK) Inhibitors market include Alfasigma Group, Asana BioSciences, LLC., HIGHLIGHTLL PHARMA, Akeso Biopharma Co., Ltd., among others.
Janus Kinase (JAK) Inhibitors Market Key Development
In July 2024, the U.S. Food and Drug Administration approved LEQSELVI (deuruxolitinib) for treating adults with severe alopecia areata. LEQSELVI is an oral Janus Kinase (JAK) inhibitor developed by Sun Pharmaceutical Industries Limited and is available at a dose of 8mg tablets.
In September 2023, the U.S. Food and Drug Administration approved OJJAARA (momelotinib), a Janus Kinase (JAK) inhibitor for treating myelofibrosis patients with anemia. Ojjaara is a once-a-day, oral JAK1/JAK2 and activin A receptor type 1 (ACVR1) inhibitor developed by GSK, and can treat anemia, constitutional symptoms, and splenomegaly in myelofibrosis patients.
Market Scope
Metrics | Details | |
CAGR | 6.8% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Product | Abrocitinib, Baricitinib, Delgocitinib, Fedratinib, Ruxolitinib, Tofacitinib, Upadacitinib, and Others |
Indication | Reversible Inhibitors and Irreversible Inhibitors | |
Distribution Channel | Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Eczema, Alopecia Areata, Myelofibrosis, Ulcerative Colitis, Graft-Versus-Host Disease (GVDH), Polycythemia Vera, and Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |