Market Size
The Global Hunter Syndrome Treatment Market reached US$ 747.06 million in 2024 and is expected to reach US$ 1,417.79million by 2033, growing at a CAGR of 7.5% during the forecast period 2025-2033.
Hunter syndrome, also known as Mucopolysaccharidosis Type II (MPS II), is a rare, inherited genetic disorder. It occurs when the body is unable to break down certain complex sugars, leading to their abnormal accumulation in various tissues. This buildup causes progressive damage to organs and tissues, resulting in a range of physical and cognitive impairments.
The symptoms of the syndrome typically appear in early childhood, and they can include developmental delays, hearing loss, joint stiffness, and respiratory issues. Over time, the condition can severely impact a child's growth and overall quality of life. Since it is inherited in an X-linked recessive manner, it primarily affects males, while females are usually carriers.
There are three drugs approved as of 2025 in specific regions as follows:
Disease Name | Common Name | Approved Drug | Country |
| MPS II | Hunter Syndrome | Idursulfase (ELAPRASE) | United States, Europe, Japan, and Mexico |
| IZCARGO | Japan | ||
| Hunterase ICV | Republic of Korea, Russia, Japan |
For more details on this report – Request for Sample
Executive Summary
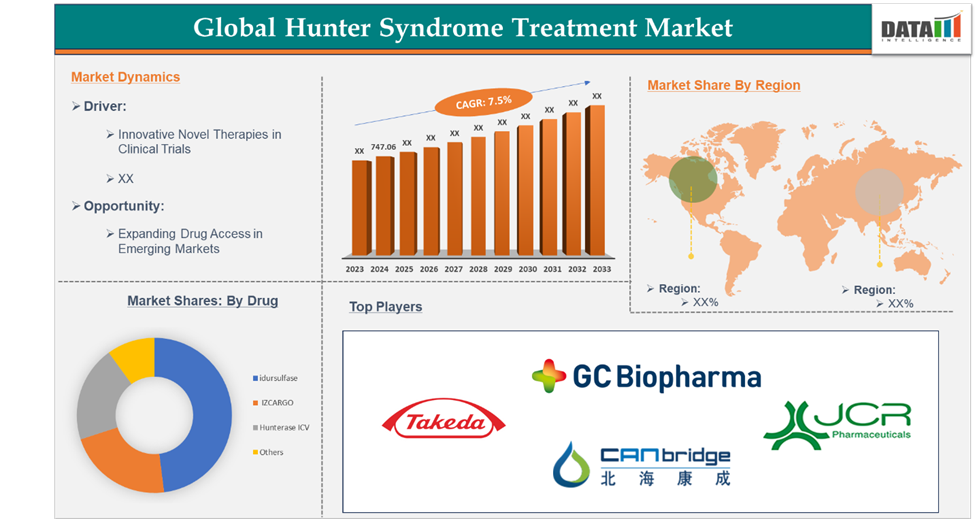
Market Dynamics: Drivers & Restraints
Innovative Novel Therapies in Clinical Trials
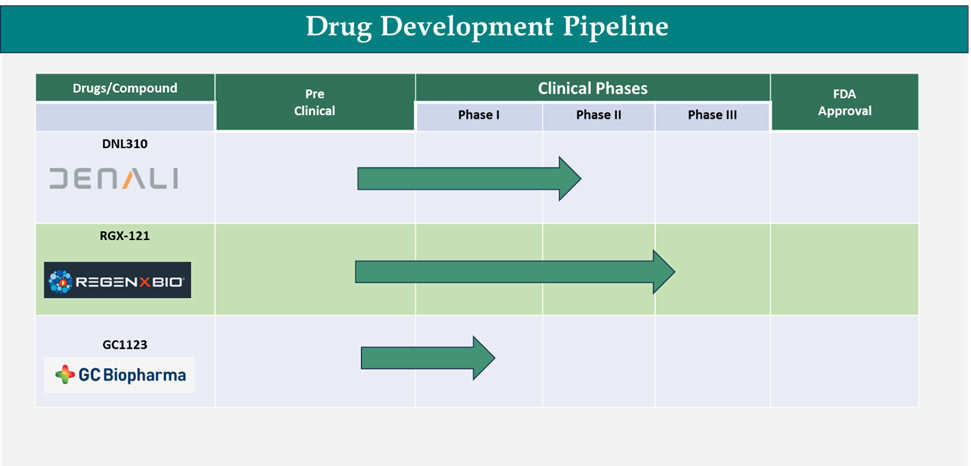
The ongoing clinical trials for innovative therapies are poised to have a major impact on the growth of the Hunter syndrome treatment market. As new treatments progress through clinical stages, they bring the potential for more effective, targeted therapies that could significantly improve patient outcomes. These clinical trials are crucial in determining the safety, efficacy, and long-term benefits of treatments such as gene therapies and next-generation enzyme replacement therapies (ERTs). For instance, in September 2024, REGENXBIO announced positive results from the Phase I/II/III CAMPSIITE trial of RGX-121 for treating Mucopolysaccharidosis Type II (Hunter syndrome). The results will be presented at the 2024 SSIEM Annual Symposium. These support RGX-121 as a potential one-time gene therapy for MPS II, with the possibility of becoming the first treatment to address the neurocognitive decline and serve as a first-line option for patients with neuronopathic disease in the U.S. If successful, these therapies could not only slow disease progression but also provide more sustainable, long-term solutions, reducing the frequency and duration of treatment resulting in the overall market growth.
Additionally, successful clinical trial outcomes may result in more competitive pricing and greater accessibility, especially in regions where current therapies are financially restrictive. The insights gained from clinical trials will further shape treatment protocols and open up new avenues for managing Hunter syndrome, ultimately accelerating market growth. As the pipeline of therapies moves forward, the promise of more effective treatments and the expansion of options will fuel the continued evolution of the Hunter syndrome treatment market.
Below is the representation of key pipeline drugs that are in various clinical phases of Hunter syndrome treatment.
High Treatment Costs
The high cost of treatment poses a significant challenge to the growth of the Hunter syndrome treatment market. Enzyme replacement therapies (ERTs) like idursulfase, while effective, require lifelong administration, which makes the overall treatment financially burdensome for patients and healthcare systems. This long-term treatment requirement, coupled with high treatment costs, creates a barrier for patients with lower incomes. The financial strain limits access to care, particularly in low- and middle-income countries with limited healthcare budgets, potentially leading to delayed treatments or the inability to access the most effective therapies, which can even affect the market growth.
Market Segment Analysis
The global Hunter syndrome treatment market is segmented based on drug and region.
Idursulfase in the drug segment is dominating the market.
The idursulfase is expected to continue its dominance in the Hunter syndrome treatment market, largely due to its proven effectiveness as an enzyme replacement therapy (ERT) for mucopolysaccharidosis type II (MPS II). As a recombinant version of the enzyme iduronate-2-sulfatase, which is deficient in MPS II patients, idursulfase has shown significant clinical benefits.
Additionally, the treatment’s long-term safety and ability to slow disease progression have made it the standard of care for MPS II. These established benefits ensure that idursulfase continues to dominate the market, with patients and healthcare providers relying on it for the effective management of Hunter syndrome.
Market Geographical Analysis
North America dominated the Hunter Syndrome Treatment market.
The North America region has the highest market share in the global hunter syndrome treatment market. A significant contributor to this dominance is the presence of major biopharmaceutical companies in the region, many of which are focused on developing cutting-edge treatments for Hunter syndrome.
The key companies have a robust pipeline of promising therapies in various stages of development, offering hope for more effective treatment options in the future. For instance, in February 2025, Denali Therapeutics announced the primary analysis of its Phase 1/2 study on tividenofusp alfa (DNL310) in 47 Hunter syndrome (MPS II) participants, covering a 24-week treatment period and long-term follow-up. With the recent Breakthrough Therapy designation, Denali plans to submit a biologics license application (BLA) in early 2025 for accelerated approval, aiming to deliver the treatment to the Hunter syndrome community by late 2025 or early 2026.
Thus, the above factors are expected to hold North America in the dominant position and will continue to play a crucial role in the ongoing progress of the market.
Global Market Players
The global market players in the Hunter Syndrome treatment market are Takeda Pharmaceuticals U.S.A., Inc., GC Biopharma, CANbridge Life Sciences Ltd, and JCR Pharmaceuticals Co., Ltd., among others.
Key Developments
- In January 2025, Denali Therapeutics announced that the U.S. FDA has granted a Breakthrough Therapy Designation for tividenofusp alfa (DNL310) to treat Hunter syndrome (MPS II). This adds to the existing fast-track, Orphan Drug, and Rare Pediatric Disease designations already granted for the therapy.
- In April 2023, JCR Pharmaceuticals and Sumitomo Pharma entered into a co-promotion agreement for IZCARGO I.V. Infusion 10 mg in Japan. IZCARGO is a recombinant treatment for mucopolysaccharidosis type II, currently marketed by JCR.
- In December 2022, JCR Pharmaceuticals announced that the U.S. FDA has granted Rare Pediatric Disease Designation (RPDD) to JR-141 (pabinafusp alfa) for the treatment of Mucopolysaccharidosis type II (Hunter syndrome).
| Metrics | Details | |
| CAGR | 7.5% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Mn) | |
| Segments Covered | Drug | Idursulfase, IZCARGO, Hunterase ICV, Others |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials and product pipelines and forecasts upcoming pharmaceutical advancements.
- Type Performance & Market Positioning: Analyze product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: This covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyze competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient Type delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global hunter syndrome treatment market report would provide approximately 45 tables, 46 figures, and 180 pages.
Target Audience 2024
- Manufacturers: Pharmaceutical, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.