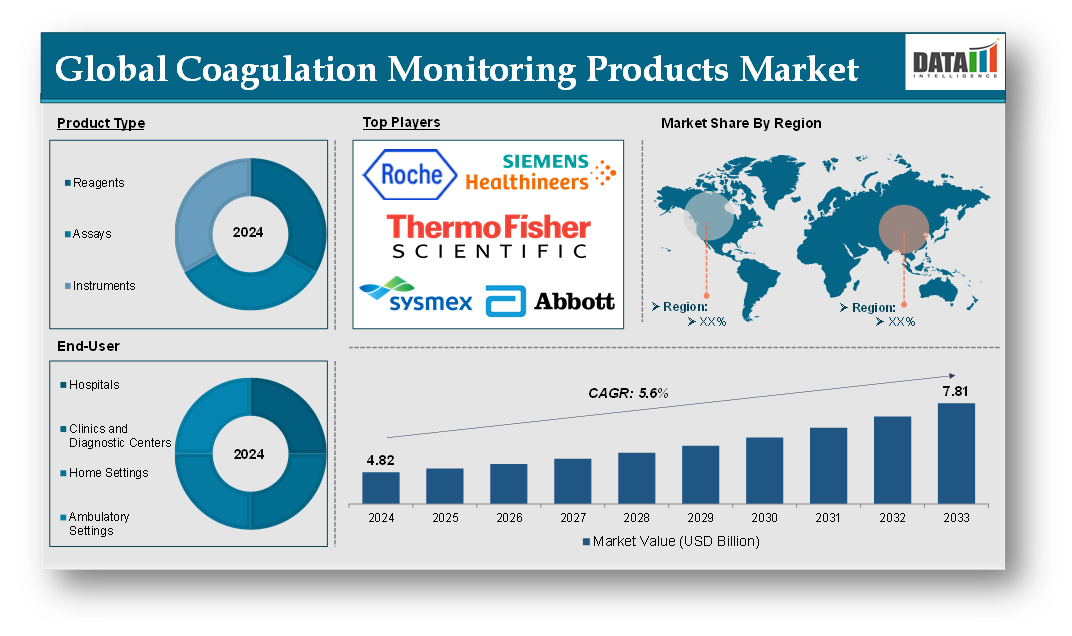
Market Size
The Global Coagulation Monitoring Products Market reached US$ 4.82 billion in 2024 and is expected to reach US$ 7.81 billion by 2033, growing at a CAGR of 5.6% during the forecast period 2025-2033.
Coagulation monitoring products are medical devices and diagnostic tools designed to measure and evaluate the coagulation (clotting) ability of blood. These products are used primarily to monitor the effectiveness of anticoagulation therapy (such as for patients on warfarin or other blood thinners) or to assess the risk of bleeding and thrombosis.
Coagulation monitoring helps in diagnosing and managing disorders related to blood clotting, including conditions like deep vein thrombosis (DVT), pulmonary embolism (PE), atrial fibrillation (AF) and hemophilia. The most common use of coagulation monitoring products is to manage patients on anticoagulant drugs like warfarin, heparin and the newer direct oral anticoagulants. These tests help adjust medication doses to ensure therapeutic efficacy and minimize the risk of adverse events, such as bleeding or clot formation.
The coagulation monitoring products market has grown significantly over the last few years due to increased awareness of the importance of coagulation monitoring in both clinical and home settings, rising incidences of coagulation disorders and technological advancements in point-of-care devices.
For instance, according to the National Institute of Health study, extracorporeal membrane oxygenation (ECMO) patients are typically unwell, which raises the risk of bleeding problems. The bleeding rate during ECMO ranges from 20.8% to 39.6%, with the cannula site (13.2%), gastrointestinal tract (5.5%), lungs (6.1%) and central nervous system (3.9%) being the most common sites. ECMO patients are also susceptible to thrombosis problems such as ischemic stroke, right ventricular thrombus, left ventricular thrombus, and pulmonary embolism. Thus, this risk of bleeding complications increases the demand for coagulation monitoring products.
Executive Summary
For more details on this report – Request for Sample
Market Dynamics: Drivers & Restraints
Rising prevalence of blood disorders
The rising prevalence of blood disorders is significantly driving the growth of the coagulation monitoring products market and is expected to drive the market over the forecast period. The rising prevalence of blood disorders, particularly coagulation-related conditions such as venous thromboembolism (VTE), atrial fibrillation (AF), hemophilia and vitamin K deficiencies, is significantly driving the coagulation monitoring products market. These disorders require continuous monitoring of blood clotting and anticoagulation therapy to prevent severe complications such as stroke, organ damage and excessive bleeding.
For instance, according to the Centers for Disease Control and Prevention (CDC), up to 900,000 people in the United States are affected by venous thromboembolism (VTE, a blood clot), each year. An estimated 60,000-100,000 Americans die of VTE each year and many others have long-term complications from VTE. Sudden death is the first symptom in about one-quarter (25%) of people who have a pulmonary embolism. The precise number of people affected by either deep vein thrombosis (DVT) or pulmonary embolism (PE) is unknown, although as many as 900,000 people could be affected each year in the United States.
Blood disorders like atrial fibrillation (AF) and deep vein thrombosis (DVT) often require long-term anticoagulation therapy such as warfarin, heparin and DOACs to prevent clot formation. These treatments require frequent coagulation tests to monitor the International Normalized Ratio (INR), a key measure of clotting, to ensure the therapy is within the therapeutic range.
For instance, according to the National Institute of Health, atrial fibrillation has been estimated that 6-12 million people will suffer from this condition in the US by 2050 and 17.9 million people in Europe by 2060. The worldwide prevalence of atrial fibrillation is 37,574 million cases (0.51% of the worldwide population), which increased by 33% during the last 20 years, with many requiring ongoing anticoagulation therapy, thereby increasing demand for coagulation monitoring products.
Blood cancers, such as leukemia, lymphomas and myeloma, can affect the production of platelets, clotting factors and other key components of the coagulation system. This leads to an increased risk of bleeding or thrombosis in these patients. For instance, leukemia patients can experience thrombocytopenia (low platelet count), making them more susceptible to bleeding or clotting complications.
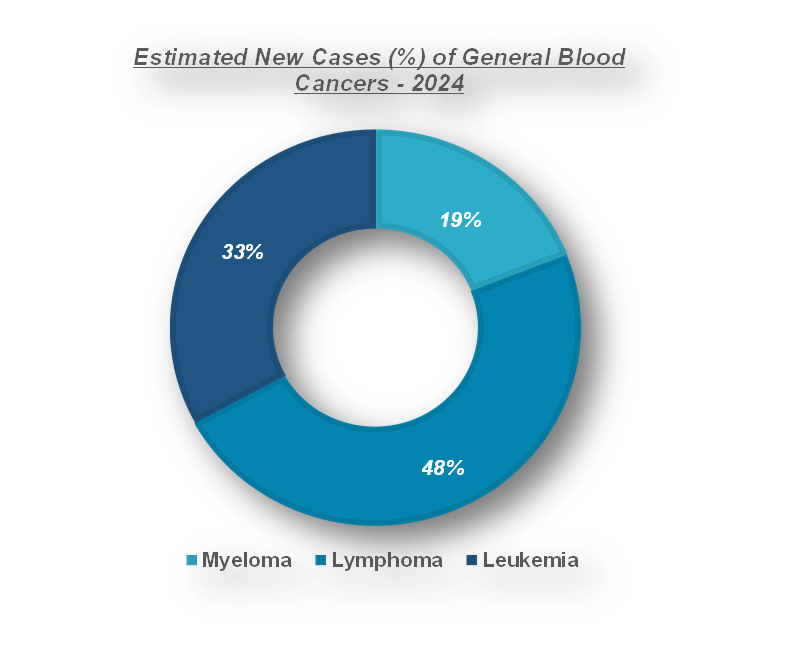
According to the Leukemia & Lymphoma Society, approximately every 3 minutes, one person in the US is diagnosed with leukemia, lymphoma or myeloma. An estimated combined total of 187,740 people in the US are expected to be diagnosed with leukemia, lymphoma or myeloma in 2024. New cases of leukemia, lymphoma and myeloma are expected to account for 9.4 percent of the estimated 2,001,140 new cancer cases that will be diagnosed in the US in 2024.
Competition from alternative diagnostic methods
Competition from alternative diagnostic methods is expected to hamper the growth of the coagulation monitoring products market over the forecast period. While traditional coagulation tests and point-of-care devices are widely used for monitoring blood clotting disorders, alternative diagnostic methods, such as non-invasive technologies, biosensors and wearable devices, are emerging as competitors, offering advantages in terms of cost, ease of use and patient comfort. These alternatives pose a challenge to the market by potentially reducing the reliance on traditional coagulation monitoring methods.
The advent of wearable devices, such as smartwatches and specialized biosensors, capable of tracking biomarkers associated with coagulation disorders is a growing concern for the coagulation monitoring products market. These devices are increasingly able to monitor various health parameters, including blood pressure, heart rate and potentially even blood clotting, with minimal user intervention.
For instance, companies like Apple are exploring the integration of health-monitoring capabilities in their wearables, which could potentially extend to monitoring blood parameters like clotting, thus reducing the demand for traditional coagulation monitoring systems.
While traditional coagulation monitoring devices can be expensive for individual patients, particularly for home care use, alternative diagnostic methods, such as biosensors and wearable health devices are increasingly becoming more affordable and accessible. This is particularly important in low-resource settings, where cost is a major barrier to the adoption of traditional coagulation monitoring systems.
Market Segment Analysis
The global coagulation monitoring products market is segmented based on product type, end-user and region.
Product Type:
The reagents segment is expected to dominate the coagulation monitoring products market share
Coagulation reagents are specialized products used in diagnostic testing to evaluate blood clotting mechanisms. Reagents are used in tests like prothrombin time, aPTT, and fibrinogen, detecting clotting factor deficiencies, anticoagulant therapy efficacy and bleeding disorders. These reagents are essential in hematology, particularly in health screening, illness diagnosis and the treatment of blood clotting disorders.
Advancements in reagent formulations have improved test sensitivity, stability and compatibility with automated analyzers. The demand for high-quality, standardized reagents is driven by the increasing prevalence of coagulation disorders, growing surgical procedures, and the adoption of point-of-care testing devices.
Coagulation reagents are essential components in diagnostic procedures, specifically for determining the blood's capacity to clot properly. They play an important role in a variety of medical settings, including routine health screenings and emergency medicine. Commonly used coagulation reagents include PT Reagents, aPPT Reagents, Thrombin Time Reagents, D-Dimer Reagents and Others.
Market Geographical Share
North America is expected to hold a significant position in the coagulation monitoring products market share
The North America region is expected to hold the largest market share over the forecast period owing to the presence of major market players particularly in the United States such as F. Hoffmann-La Roche Ltd., Siemens Healthcare AG, Abbott Laboratories and other startups and emerging players. These major market players mainly focus on market expansion by novel product launches, advancements in the products, expansion strategies to enhance the company’s product portfolio for coagulation monitoring, which is expected to further drive the market growth.
For instance, in February 2024, Roche launched three new coagulation tests for the oral Factor Xa inhibitors apixaban, edoxaban and rivaroxaban. These anticoagulants were added to the World Health Organization's Model List of Essential Medicines due to their potential advantages for patients. The tests could aid in clinical decision-making in patients receiving direct oral anticoagulants for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation, coronary artery disease (CAD), peripheral arterial disease (PAD) and venous thromboembolism (VTE).
Additionally, in July 2023, Baxter International launched the PERCLOT Absorbable Hemostatic Powder in the U.S., a passive, absorbable powder designed for patients with intact coagulation to address mild bleeding.
Moreover, the rising prevalence of blood disorders in the region especially in the United States is driving the demand for coagulation monitoring products market. For instance, according to Leukemia & Lymphoma Society, about every 3 minutes, a person in the US is diagnosed with either leukemia, lymphoma or myeloma. In the year 2024, the overall projected case of leukemia, lymphoma or myeloma in the US is 187,740. New incidences of leukemia, lymphoma and myeloma cases will represent 9.4% of the new cancer cases projected for diagnosis in the US in the year 2024 which will be 2,001,140 cases.
Asia-Pacific is growing at the fastest pace in the coagulation monitoring products market
The Asia-Pacific region is experiencing the fastest growth in the coagulation monitoring products market. This is due to a rise in the cases of blood disorders and also non-communicable diseases that require monitoring of blood coagulation such as diabetes and cardiovascular diseases. Other factors stimulating the growth of the market are change in people’s perception towards the attention that needs to be given
to the diagnosis and treatment of various complications arising from blood coagulability. Improvements in health care systems and more adoption of diagnostic products and services are also driving the growth of the market.
For instance, in September 2024, Sysmex Corporation has officially dispensed the HISCL HIT IgG Assay Kit in Japan which measures IgG antibody levels of platelet factor 4 and heparin complexes. The kit is for use in Automated Blood Coagulation Analyzers CN-6500/CN-3500 which include HISCL-Series technology.
Additionally, in May 2023, QuidelOrtho and Render launched the IHTEG6 thromboelastography at the 20th China Association of Clinical Laboratory Practice Expo. Thromboelastography is a crucial tool for monitoring coagulation during surgery and has become a vital tool for managing blood products in sophisticated nations worldwide.
Major Global Players
The major global players in the coagulation monitoring products market include Siemens Healthcare AG, Abbott Laboratories, Beckman Coulter, Inc., Sysmex Corporation, Thermo Fisher Scientific Inc., HemoSonics, LLC., Helena Laboratories Corporation., F. Hoffmann-La Roche Ltd., Horiba Group, Maccura Biotechnology Co., Ltd., Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Sclavo Diagnostics International SpA, BIOGENIX INC. PVT. LTD. and among others.
Market Scope
| Metrics | Details | |
| CAGR | 5.6% | |
| Market Size Available for Years | 2018-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Mn) | |
| Volume (Units) | ||
| Segments Covered | Product Type | Reagents, Assays and Instruments |
| End-User | Hospitals, Clinics and Diagnostic Centers, Home Settings and Ambulatory Settings | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials, product pipelines, and forecasts upcoming advancements in medical devices and pharmaceuticals.
- Product Performance & Market Positioning: Analyzes product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: Covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyzes competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient product delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global coagulation monitoring products market report delivers a detailed analysis with 63 key tables, more than 46 visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2025
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.