Bi-Specific MAbs Market Size& Industry Outlook
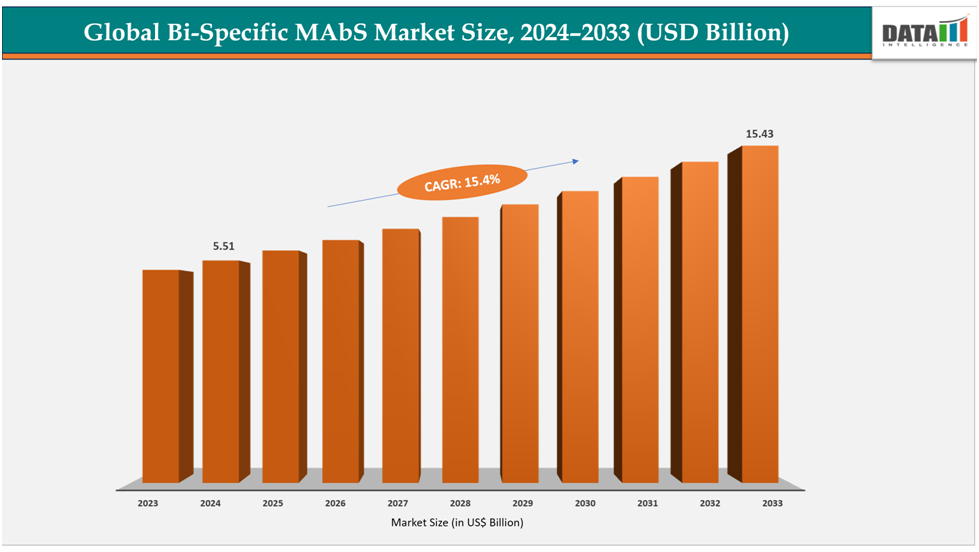
The global Bi-Specific MAbs market size reached US$ 4.82Billion with rise of US$5.51Billion in 2024 is expected to reach US$ 15.43 Billion by 2033, growing at a CAGR of 15.4%during the forecast period 2025-2033.
One of major growing factor in this market is rapid technological advances in gene-editing and cell engineering are transforming the Bi-Specific MAbs market by making treatments more precise, effective, and scalable. Breakthrough tools such as CRISPR/Cas9, TALENs, and zinc-finger nucleases enable highly accurate editing of cancer-related genes, minimizing off-target effects and improving safety.
At the same time, innovations in cell engineering such as next-generation CAR-T and TCR-T therapies are enhancing immune cells to better recognize and destroy tumors, resist immune suppression, and persist longer in the body. Furthermore, progress in viral and non-viral vector systems has improved gene delivery efficiency, while automation and advanced biomanufacturing platforms are streamlining production, reducing costs, and accelerating commercialization.
Key Highlights
North Americadominatesthe Bi-Specific MAbsmarket with the largest revenue share of 43.5% in 2024.
The Asia Pacific is the fastest-growingregion and is expected to grow at the fastest CAGR of8.1% over the forecast period.
Based on drug type, Blinatumomabsegmentled the market with the largest revenue share of 34.1% in 2024.
The major market players in the Amgen, Genentech / Roche, Genmab, Janssen Biotech, Immunocore Limitedand among others.
Market Dynamics
Drivers: The rising investment, partnerships, and product approvals is significantly driving the Bi-Specific MAbs market growth
One of the biggest drivers of the bispecific antibodies market is the growing number of people suffering from chronic diseases, especially cancer, autoimmune disorders, and blood-related conditions. The global cancer burden is increasing every year, with millions of new cases reported, while autoimmune diseases such as rheumatoid arthritis and multiple sclerosis are also becoming more common.
This rising disease prevalence creates a strong demand for advanced therapies that can target diseases more effectively and with fewer side effects. Bispecific antibodies, with their ability to engage immune cells directly and attack diseased cells, are well-suited to meet this urgent medical need, making the rise of chronic diseases a major force fueling market growth.
For instance, according to National Center for Chronic Disease Prevention and Health Promotion, The US has 129 million people with at least one major chronic disease, including heart disease, cancer, diabetes, obesity, and hypertension. Five of the top 10 leading causes of death in the US are associated with preventable and treatable chronic diseases. An increasing proportion of Americans are dealing with multiple chronic conditions, with 42% having two or more and 12% having at least five. Chronic diseases also significantly impact the US healthcare system, with 90% of annual $4.1 trillion expenditure attributed to managing and treating these conditions.
Restraints: Regulatory complexity and safety/immune risks are hampering the growth of the Bi-Specific MAbs market
One of the biggest challenges facing the bispecific antibodies market is the difficulty of manufacturing these advanced drugs. Unlike traditional monoclonal antibodies, bispecifics are structurally more complex, which makes them harder to design, produce, and scale up. They often require specialized technology, higher production costs, and careful quality control to ensure safety and effectiveness. These hurdles can slow down development timelines, increase costs for companies, and limit patient access. Until manufacturing processes become more streamlined and cost-efficient, this challenge will continue to hold back the full potential of the bispecific antibody market.
For more details on this report – Request for Sample
Segmentation Analysis
The global Bi-Specific MAbs market is segmented based on drug type, indication, route of administration, distribution channel, and region.
Drug Type:
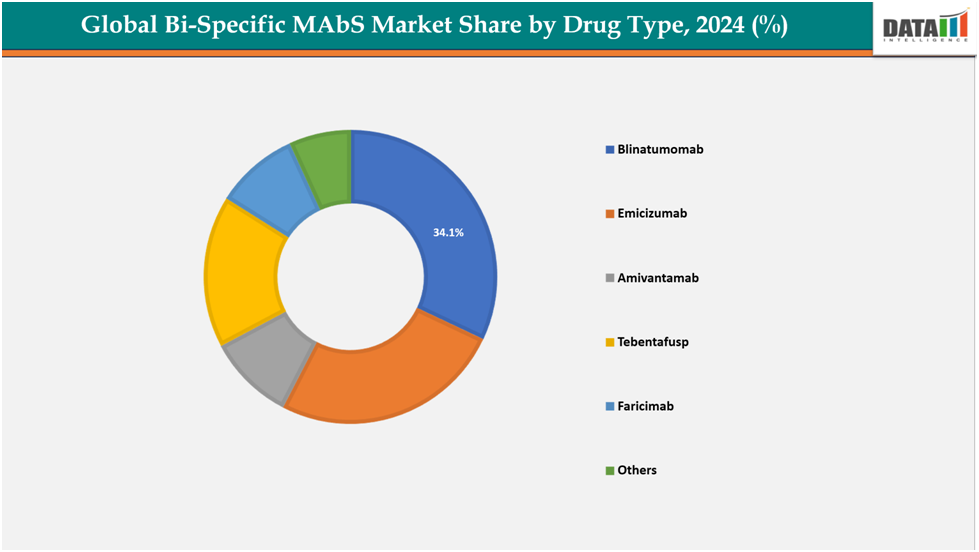
The blinatumomab from drug type segment to dominate the Bi-Specific MAbs market with a 34.1% share in 2024
Blinatumomab is one of the first bispecific antibodies which has shown strong success in treating certain types of blood cancers, such as acute lymphoblastic leukemia (ALL). Its early market entry gives it a major advantage, as doctors already have experience using it, and patients have seen positive results. Continuous clinical trials are also exploring its potential in other blood cancers, which expands its future market reach. Its proven effectiveness, established regulatory approvals, and growing use in real-world settings make Blinatumomab a key driver of the global bispecific antibody market.
For instance, in February 2025, Blinatumomab, the first bispecific T-cell engager (BiTE) antibody approved for oncology, is a significant breakthrough in treating B-cell malignancies. Its development, mechanism of action, clinical efficacy, and impact on targeted cancer therapies are reviewed, highlighting its significant role in immunotherapy advancements.
Indication: The oncology segment is estimated to have a 31.45% of the Bi-Specific MAbs market share in 2024
The oncology segment is the largest and fastest-growing application area for bispecific antibodies. Rising cancer cases worldwide, along with the urgent need for treatments that are more precise and less toxic than traditional chemotherapy, are driving demand. Bispecific antibodies are especially powerful in oncology because they can guide immune cells directly to cancer cells, boosting treatment effectiveness. Pharmaceutical companies are heavily investing in this area, with most ongoing clinical trials focusing on solid tumors and blood cancers. This strong research pipeline, combined with high patient need, ensures that oncology remains the leading force behind market growth.
For instance, in August 2025, Alphamab Oncology has received approval for its innovative bispecific antibody-drug conjugate, JSKN022, for the treatment of advanced malignant solid tumors. The IND application has been accepted by the NMPA's Center for Drug Evaluation, and the company plans to initiate a first-in-human clinical study.
Geographical Analysis
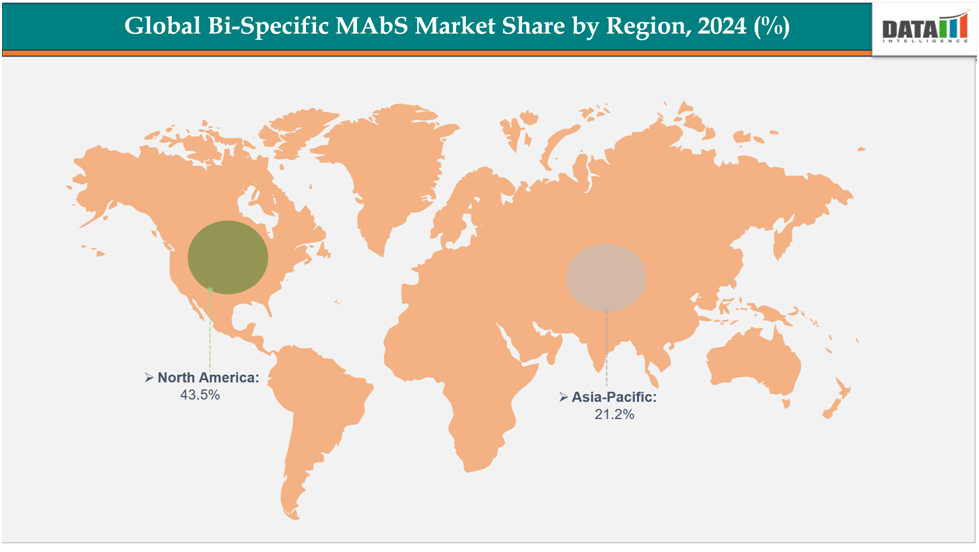
North America dominates the global Bi-Specific MAbs market with a 43.5% in 2024
North America is one of the most important regions for the bispecific antibodies market. The area has world-leading research centers, strong pharmaceutical companies, and major funding from both private investors and governments. Cancer and autoimmune diseases are highly prevalent here, which creates a strong need for advanced therapies like bispecific antibodies.
The United States dominates within North America thanks to its massive biotech industry and advanced R&D ecosystem. American universities, biotech startups, and big pharmaceutical companies are all investing heavily in bispecific antibody research, especially for cancer treatment. The U.S. Food and Drug Administration (FDA) has also become more open to approving breakthrough therapies, which shortens the time it takes to bring new drugs to patients. In addition, the country sees a high number of partnerships, mergers, and licensing agreements that strengthen innovation and expand drug pipelines.
For instance, in August 2025, RemeGen Co., Ltd. has received FDA clearance for phase II clinical trials of its bispecific antibody, RC148, for treating advanced malignant solid tumors in the US.
Europe is the second region after North America which is expected to dominate the global Bi-Specific MAbs market with a 34.5% in 2024
Europe is another strong region for bispecific antibodies, with major biotech hubs in countries like the UK, France, and Switzerland. The European Union has streamlined regulatory pathways that help companies test and launch advanced therapies more efficiently. Collaboration is a key strength in Europe, as many projects are run jointly by academic research institutes and pharmaceutical companies.
Within Europe, Germany plays a central role in driving this market. The country has a highly developed pharmaceutical industry, world-class research institutes, and significant funding for oncology research. German companies are investing in advanced antibody platforms and often collaborate with global pharma players. The country also benefits from modern manufacturing infrastructure that supports large-scale production of biologics, including bispecific antibodies.
For instance, in July 2025, BioMed X and Servier have established the first XSeed Labs in Europe, utilizing a crowdsourcing model to integrate academic researchers into pharmaceutical R&D facilities. The project aims to develop an AI-powered platform for designing bispecific antibodies.
The Asia Pacific region is the fastest-growing region in the global Bi-Specific MAbs market ,with a CAGR of 8.1% in 2024
The Asia-Pacific region is experiencing the fastest growth in the bispecific antibodies market. One of the main drivers is its large and diverse patient population, combined with rapidly rising cases of cancer and chronic diseases. Countries like China, India, South Korea, and Australia are investing heavily in biotechnology research and advanced drug development. China, in particular, has seen a surge of local biotech companies focusing on bispecific antibody pipelines, supported by government incentives.
Japan is a mature market with unique strengths. The country faces a rapidly aging population, which increases the need for innovative cancer and immune therapies. Japanese pharmaceutical companies are strong in biologics development and often collaborate with global partners to accelerate innovation.
For instance, in March 2025, Chugai Pharmaceutical Co., Ltd. has launched LUNSUMIO, an antineoplastic agent and anti-CD20/CD3 humanized bispecific antibody, for treating relapsed or refractory follicular lymphoma patients who have received two or more standard therapies, with the generic name being mosunetuzumab (genetical recombination).
Competitive Landscape
Top companies in the Bi-Specific MAbs market include Amgen, Genentech / Roche, Genmab, Janssen Biotech, Immunosera Limited and among others.
Amgen: Amgen, a leading player in the bispecific T-cell engager (BiTE) market, has established a significant revenue foothold and validated bispecific as a therapeutic class. BLINCYTO, the first approved BiTE, has been commercialized and has been used in clinical and commercial settings. Amgen has also invested heavily in next-generation, half-life-extended BiTE programs, such as AMG-701 and AMG-160. Despite facing clinical and development setbacks, such as toxicity and delivery challenges, Amgen remains a major innovator and market leader in the global bispecific-antibody landscape. The company continues to expand indications for the agent.
Key Developments:
In July 2025, Akeso, Inc announced that it has successfully enrolled the first patient in its Phase Ia clinical trial of AK146D1, a bispecific Antibody-Drug Conjugate (ADC) designed to target Trop2 and Nectin4. This marks the Company’s first bispecific ADC to advance into clinical development.
In June 2025, BioNTech SE and Bristol Myers Squibb announced a global agreement to jointly develop and commercialize BioNTech’s investigational bispecific antibody, BNT327, for multiple solid tumor types. Through this collaboration, the two companies will work together to expand and accelerate the clinical development of this promising candidate.
Market Scope
Metrics | Details | |
CAGR | 15.4% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Drug Type | Blinatumomab, Emicizumab, Amivantamab, Tebentafusp, Faricimab, Others |
Indication | Oncology, Immunology/Autoimmune, Hematology, Ophthalmology, Rare diseases, Infectious diseases | |
Route of Administration | Intravenous (IV), Subcutaneous (SC) | |
Distribution Channel | Hospital Pharmacies, Retail Pharmacies | |
Regions Covered | North America, Europe, Asia-Pacific, South America and the Middle East & Africa | |
The global Bi-Specific MAbs market report delivers a detailed analysis with 62 key tables, more than 57visually impactful figures, and 159 pages of expert insights, providing a complete view of the market landscape.