Induced Pluripotent Stem Cell (iPS Cell) Market Size
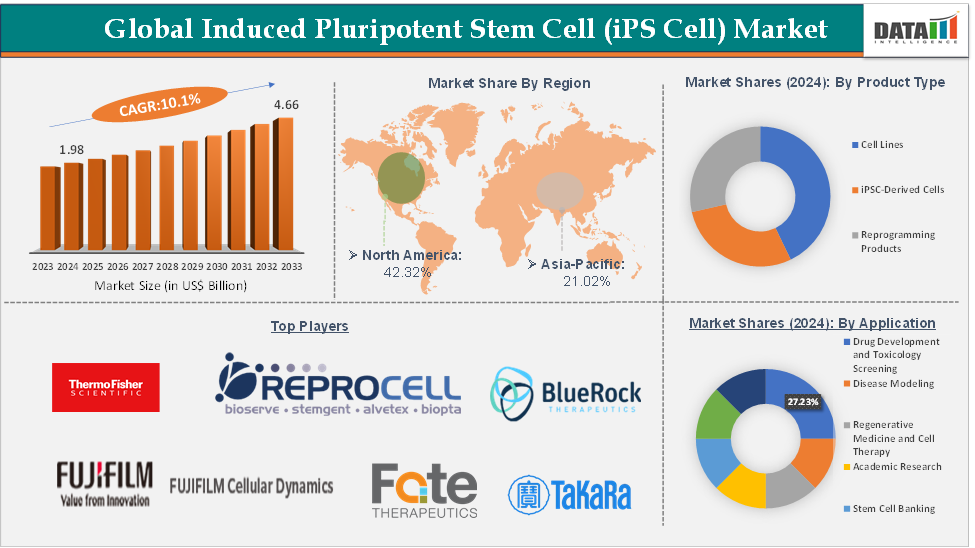
The global induced pluripotent stem cell (iPS Cell) market size reached US$ 1.98 Billion in 2024 from US$ 1.81 Billion in 2023 and is expected to reach US$ 4.66 Billion by 2033, growing at a CAGR of 10.1% during the forecast period 2025-2033.
Induced Pluripotent Stem Cell (iPS Cell) Market Overview
The induced pluripotent stem cell market is witnessing robust growth fueled by advancements in regenerative medicine, drug discovery, and personalized healthcare. iPSCs, first developed in 2006, revolutionize the field by enabling the reprogramming of adult somatic cells into pluripotent stem cells without the ethical concerns associated with embryonic stem cells. This breakthrough has unlocked unprecedented opportunities across multiple sectors, from disease modeling to cell therapy.
The iPSC market stands at a transformative stage with strong potential to reshape drug development and regenerative therapies. While challenges related to cost, safety, and regulation remain, ongoing technological and scientific breakthroughs, coupled with growing demand for personalized treatments, present significant growth opportunities. Strategic partnerships, increased funding, and evolving regulatory frameworks will be critical in unlocking the full potential of iPSC-based applications in the coming years.
Induced Pluripotent Stem Cell (iPS Cell) Market Executive Summary
Induced Pluripotent Stem Cell (iPS Cell) Market Dynamics
Drivers:
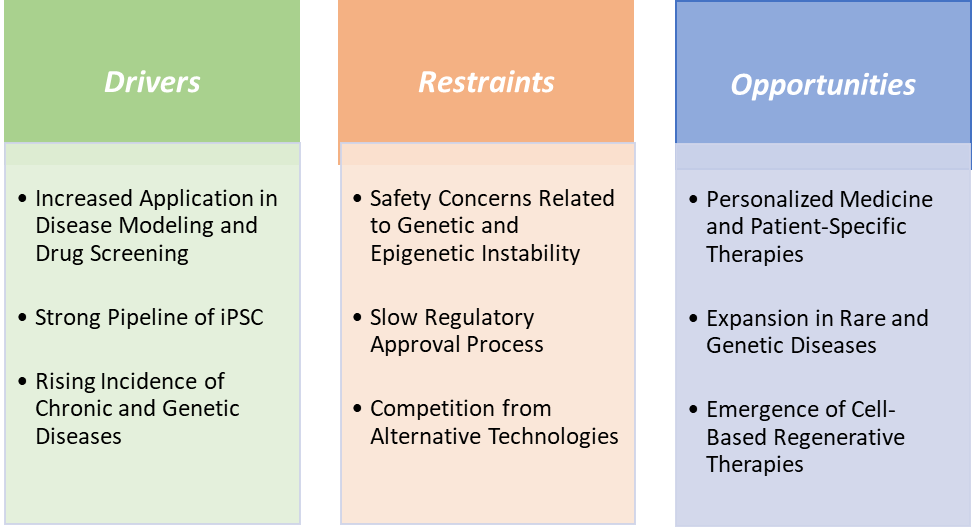
The ability of iPSCs to provide accurate, patient-specific disease models and safer, more predictive drug screening platforms is propelling investments, partnerships, and market expansion, making this application one of the strongest growth engines in the iPSC industry. iPSCs can be derived from patients with specific genetic conditions, allowing researchers to replicate complex diseases like Parkinson’s, Alzheimer’s, and inherited cardiac disorders in a dish. This overcomes limitations of traditional animal models, which often fail to mimic human pathophysiology accurately.
Pharmaceutical companies are increasingly focusing on the development of various iPSC products modelling various diseases and drug screening, which is driving the market growth. For instance, in January 2025, Ncardia launched Ncyte Microglia, a groundbreaking tool to advance research in neurodegenerative diseases and central nervous system (CNS) disorders. Ncyte Microglia are hiPSC-derived models that closely mimic primary human microglia, exhibiting key functional characteristics such as phagocytosis, cytokine signaling response, and the expression of microglial markers including IBA1, TREM2, and TMEM119. These features make Ncyte Microglia highly suitable for studying microglial behavior in disease-relevant contexts, assessing immune responses, and enabling high-throughput drug screening applications.
Similarly, in April 2025, Cellistic launched the Echo-NK platform to enable the scalable manufacturing of allogeneic cell therapies to target multiple diseases. Cellistic’s Echo-NK platform provides drug developers with a commercially viable solution for an Off-the-Shelf NK cell therapy.
A strong pipeline of iPSCs is also driving the induced pluripotent stem cells market growth
Numerous iPSC-based therapies are progressing through clinical and preclinical stages, targeting a wide range of diseases such as neurodegenerative disorders, retinal diseases, cardiovascular conditions, fertility problems, and rare genetic disorders. This growing pipeline signals increasing confidence in iPSC technology’s therapeutic potential and attracts investments.
These trials validate safety and efficacy, pushing iPSC products closer to regulatory approval and commercialization. For instance, in January 2025, Gameto announced that the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for Fertilo, to enable the launch of the first US-based Phase 3 clinical trial for an induced pluripotent stem cell (iPSC)-based therapy. This groundbreaking milestone highlights the potential of iPSC technology to address critical unmet needs in reproductive medicine.
Additionally, in January 2025, Aspen Neuroscience, Inc. advanced its plans for automating the production of ANPD001. ANPD001, an investigational cell therapy program for treating people with Parkinson’s disease (PD), is currently being studied in the company’s ASPIRO Phase 1/2a clinical trial. Designed to replace lost dopamine-producing neurons in the brain, Aspen differentiates these DANPCs from patient-specific induced pluripotent stem cells (iPSCs), derived from the patient’s skin cells.
Restraints:
Safety concerns related to genetic and epigenetic instability are hampering the growth of the induced pluripotent stem cell market
The reprogramming process used to generate iPSCs can introduce genetic mutations or chromosomal abnormalities. Additionally, extended cell culture and expansion can accumulate further mutations. These genetic instabilities raise significant safety concerns, particularly the risk of tumorigenicity (cancer formation) when iPSC-derived cells are used in clinical therapies. iPSCs can retain “epigenetic memory” from their original somatic cells, leading to incomplete reprogramming or abnormal differentiation patterns. Such epigenetic instability can cause variability in iPSC behavior, reducing reproducibility and predictability in therapeutic and research applications.
Due to these safety concerns, regulatory agencies like the FDA and EMA require extensive testing and validation before approving iPSC-based therapies, leading to longer development timelines and higher costs. These safety issues make investors and pharmaceutical companies cautious, slowing down large-scale commercialization and adoption.
Opportunities:
Expansion in rare and genetic diseases creates a market opportunity for the induced pluripotent stem cells market
iPSCs can be derived from patients with rare or genetic disorders to create personalized disease models that accurately mimic disease pathology. This enables a better understanding of disease mechanisms and the development of targeted therapies, which is especially valuable for conditions with limited existing treatment options.
According to the National Institute of Health, more than 7,000 rare diseases have been identified, highlighting their significant global impact. Over 30 million people in the United States, more than 29 million in the EU, and an estimated 400 million individuals worldwide are affected by various rare diseases, many of which lack effective therapies due to challenges in modeling disease and conducting drug testing. iPSC technology fills this gap by providing patient-specific cells for preclinical testing, accelerating drug discovery for orphan diseases.
For more details on this report – Request for Sample
Induced Pluripotent Stem Cell (iPS Cell) Market, Segment Analysis
The global induced pluripotent stem cells market is segmented based on product type, application, disease area, end-user, and region.
Drug development and toxicology screening from the application segment are expected to hold 27.23% of the market share in 2024 in the induced pluripotent stem cells market
iPSC-derived cells provide human-relevant models for assessing drug efficacy and toxicity early in the development process, reducing reliance on animal models that often fail to predict human responses accurately. This improves success rates in clinical trials and cuts down costly late-stage failures.
Major pharma firms are increasingly focusing on the development of various iPSC-based platforms and products for drug discovery, which if further accelerate the segment growth. For instance, in June 2025, iXCells Biotechnologies USA, Inc. launched its modular iPSCore platform. iXCells’ mission is to empower life science researchers and patient foundations to advance personalized therapies and accelerate the discovery of better medicines for a healthier future.
Similarly, in September 2024, BlueRock Therapeutics, a clinical stage cell therapy company and wholly owned, independently operated subsidiary of Bayer, announced the clearance of its Investigational New Drug (IND) application by the U.S. Food and Drug Administration (FDA) for OpCT-001, an investigational induced pluripotent stem cell (iPSC)-derived cell therapy for the treatment of primary photoreceptor diseases.
In January 2025, Ncardia launched Ncyte Heart in a Box, a breakthrough 3D cardiac microtissue model designed to drive innovation in cardiovascular research and drug discovery. The Ncyte Heart in a Box system is derived from hiPSCs and integrates three essential cardiac cell types: ventricular cardiomyocytes, endothelial cells, and cardiac fibroblasts. Together, these high-purity components form a physiologically relevant microtissue that replicates the complexity and functionality of the human heart.
Induced Pluripotent Stem Cell (iPS Cell) Market, Geographical Analysis
North America is expected to dominate the global induced pluripotent stem cells market with a 42.32% share in 2024
Key iPSC technology providers and contract research organizations like Fujifilm Cellular Dynamics, Thermo Fisher Scientific, REPROCELL, and other emerging market players are headquartered in North America. These companies dominate the supply of iPSC-derived cell lines, reagents, and services, and are developing various iPSC products, giving the region a competitive edge.
For instance, in May 2025, REPROCELL introduced StemEdit Human iPSC non-HLA class 1 (B2M Homo KO) and StemEdit Human iPSC non-HLA class 1/2 (B2M/CIITA Homo double KO) cell lines. These cell lines, although for research use only, were derived from StemRNA Clinical induced pluripotent stem cells (iPSCs) from a healthy donor using our StemEdit gene editing technology. These cells provide stem cell researchers with iPSCs that are devoid of HLA class 1 or both HLA Class 1 and 2 expressions, making them ideal to study the interaction of the immune system with iPSCs or cells derived from them.
Similarly, in April 2025, I Peace, Inc., a global pioneer in iPSC (induced pluripotent stem cell) technology, launched its personalized iPS cell production and longevity-focused rejuvenation therapies in the United States. iPS cell technology enables the creation of age-reversed stem cells that reflect a younger version of the individual’s biology. I Peace is now expanding the use of this breakthrough not only for regenerative medicine but also for a wide range of longevity applications.
North America’s dominance in the iPSC market stems from its strong research ecosystem, substantial funding, presence of industry leaders, advanced healthcare infrastructure, and supportive regulatory environment, which collectively accelerate innovation and market penetration.
Asia-Pacific is growing at the fastest pace in the induced pluripotent stem cells market, holding 21.02% of the market share
The Asia Pacific region is the fastest-growing market for induced pluripotent stem cells, driven by a combination of supportive government policies, robust healthcare infrastructure, and increasing investments in biotechnology research. The growing prevalence of chronic diseases and an aging population in countries like India and Japan are increasing the demand for personalized and regenerative medicine solutions, further propelling the iPSC market.
The Asia Pacific region's rapid growth in the iPSC market is attributed to strategic government investments, advancements in clinical applications, expanding healthcare infrastructure, and a rising demand for personalized medicine. These factors collectively position the region as a leader in the global iPSC market.
Induced Pluripotent Stem Cell (iPS Cell) Market Competitive Landscape
Top companies in the induced pluripotent stem cells market include Thermo Fisher Scientific Inc., REPROCELL Inc., FUJIFILM Cellular Dynamics, Axol Bioscience Ltd., Evotec, Takara Bio Inc., Fate Therapeutics, BlueRock Therapeutics LP, Aspen Neuroscience, and Ncardia, among others.
Induced Pluripotent Stem Cell (iPS Cell) Market, Key Developments
In June 2025, Pluristyx, a leading provider of tools and services for cell product development, announced the launch of its first-of-its-kind PluriForm Organoid Kit, a turnkey solution to eliminate critical bottlenecks in organoid research and allow scientists to rapidly and reliably make pluripotent aggregates using quality-assured, induced pluripotent stem cells (iPSCs). The kit saves weeks of cell culture work and eliminates variability in organoid manufacturing, allowing reproducible and iterative development and application of organoids.
In September 2024, FUJIFILM Cellular Dynamics, a leading global developer and manufacturer of human induced pluripotent stem cells (iPSCs), announced the global commercial launch of its human iPSC-derived iCell Sensory Neurons for scientists engaged in neuroscience research, drug discovery of novel pain medications, and analysis of neurotoxicity side effects.
Induced Pluripotent Stem Cell (iPS Cell) Market Scope
Metrics | Details | |
CAGR | 10.1% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Product Type | Cell Lines, iPSC-Derived Cells, and Reprogramming Products |
Application | Drug Development and Toxicology Screening, Disease Modeling, Regenerative Medicine and Cell Therapy, Academic Research, Stem Cell Banking, Gene Therapy, and Others | |
Disease Area | Neurological Disorders, Cardiovascular Diseases, Hematological Diseases, Metabolic Disorders, Infectious Diseases, Oncology, Rare Diseases, and Others | |
End-User | Academic and Research Institutes, Pharmaceutical and Biotechnology Companies, Hospitals and Clinics, Contract Research Organizations (CROs), Stem Cell Banks, and Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global induced pluripotent stem cell (iPS Cell) market report delivers a detailed analysis with 70 key tables, more than 74 visually impactful figures, and 146 pages of expert insights, providing a complete view of the market landscape.