ESR1 Mutated Metastatic Breast Cancer Diagnostics Market Size
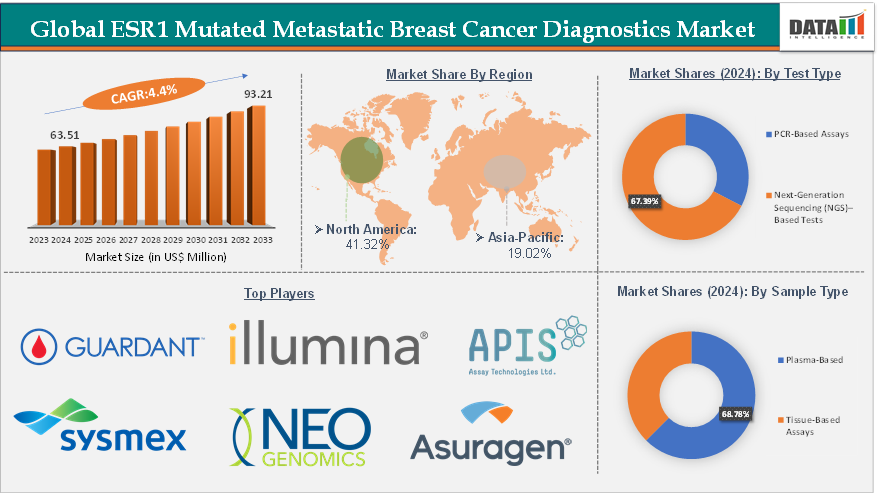
The global ESR1 mutated metastatic breast cancer diagnostics market size reached US$ 63.51 Million in 2024 from US$ 61.05 Million in 2023 and is expected to reach US$ 93.21 Million by 2033, growing at a CAGR of 4.4% during the forecast period 2025-2033.
ESR1 Mutated Metastatic Breast Cancer Diagnostics Market Overview
The ESR1-mutated metastatic breast cancer diagnostics market is an emerging and rapidly evolving segment within the broader oncology diagnostics landscape. ESR1 gene mutations, which primarily drive resistance to endocrine therapies in hormone receptor-positive (HR+) metastatic breast cancer, have become crucial biomarkers guiding treatment decisions, particularly for therapy switching to selective estrogen receptor degraders (SERDs). Companies that address current technical, reimbursement, and infrastructure barriers, while leveraging unique opportunities in serial monitoring, companion diagnostics, and emerging markets, will capture the highest value share in this niche but strategically critical segment.
ESR1 Mutated Metastatic Breast Cancer Diagnostics Market Executive Summary
ESR1 Mutated Metastatic Breast Cancer Diagnostics Market Dynamics
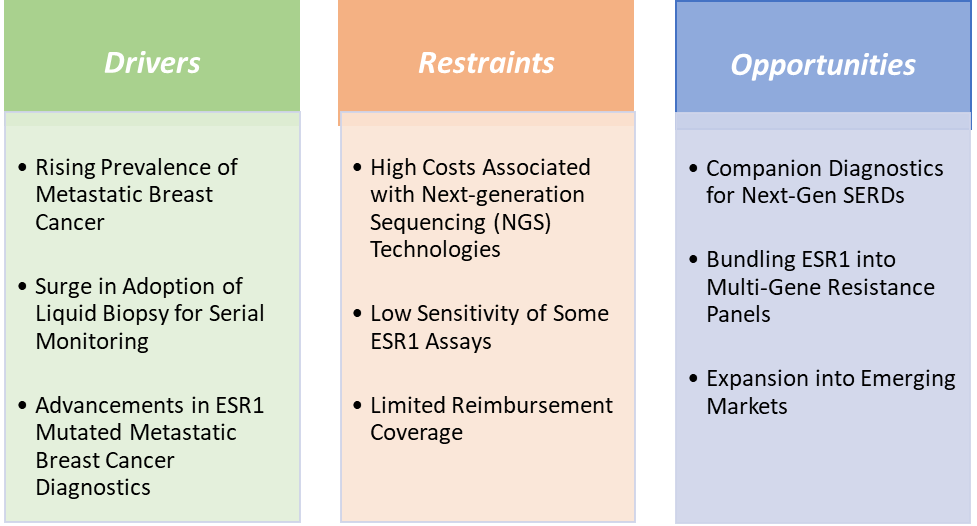
Drivers:
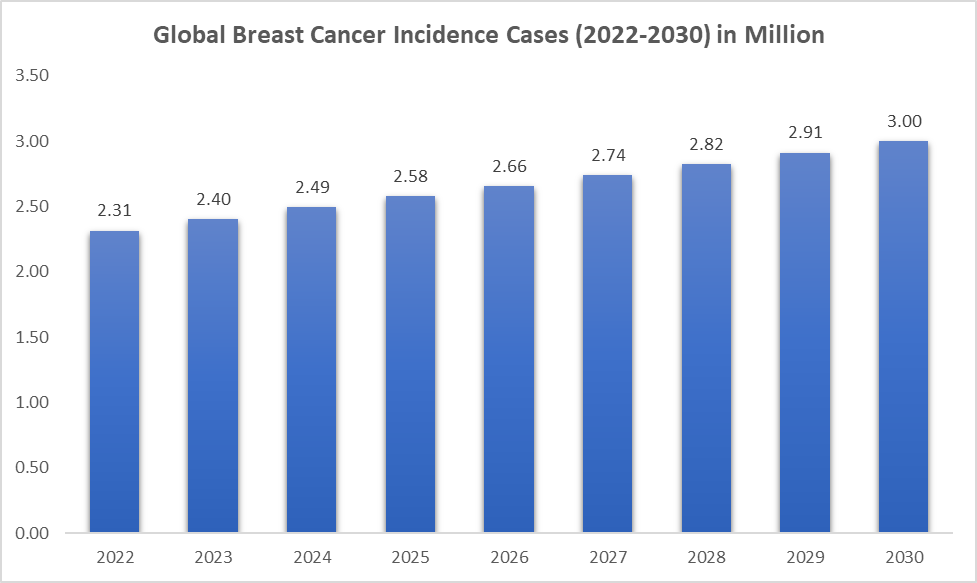
The global incidence of breast cancer has been steadily increasing due to factors such as aging populations, improved early detection (which paradoxically leads to longer survival and thus more cases progressing to metastasis), and lifestyle-related risk factors, which may pose risk to advanced metastatic breast cancers. As metastatic breast cancer becomes more common, the demand for precise and effective management tools grows accordingly.
Sources: World Health Organization | DataM Internal Estimates
For instance, according to the American Society of Clinical Oncology (ASCO), breast cancer is the second most common cause of death from cancer in women in the United States. It is estimated that 43,700 people (43,170 women and 530 men) died in the United States in 2023. In the United States, 6% of women have metastatic breast cancer when they are first diagnosed.
Additionally, according to the National Institute of Health, about 279,000 new cases of breast cancer are diagnosed in China each year, which is an increase over previous years. Also, according to the Canadian Cancer Society, 29,400 Canadian women were diagnosed with metastatic breast cancer, which represents 26% of all new cancer cases in women in 2023. 5,400 Canadian women will die from breast cancer.
The increasing prevalence of metastatic breast cancer expands the patient pool that requires ESR1 mutation, thereby significantly fueling the growth of the ESR1-mutated metastatic breast cancer diagnostics market.
Advancements in ESR1-mutated metastatic breast cancer diagnostics are also driving the market growth
Recent technological advancements in ESR1 mutation detection have significantly accelerated the growth of the ESR1-mutated metastatic breast cancer diagnostics market. Innovations such as highly sensitive liquid biopsy platforms, next-generation sequencing (NGS), and digital PCR (dPCR) enable accurate, non-invasive, and real-time monitoring of ESR1 mutations from blood samples. Thus, market players are developing highly sensitive ESR1 mutation assays, which is driving the market growth.
For instance, in December 2024, Bio-Techne Corporation announced that Asuragen, a Bio-Techne brand, launched a highly sensitive ESR1 mutation monitoring assay. ESR1 gene mutations are known to be linked to hormone receptor-positive (HR+) metastatic breast cancer. The research-use-only assay consists of a qPCR detection kit as well as an isolation kit for cell-free DNA and exosomal RNA.
Restraints:
Low sensitivity of some ESR1 assays is hampering the growth of the ESR1-mutated metastatic breast cancer diagnostics market
Accurate detection of ESR1 mutations, especially at low allele frequencies in circulating tumor DNA (ctDNA), is critical for the timely identification of endocrine therapy resistance in metastatic breast cancer patients. However, many conventional ESR1 assays, particularly older PCR-based tests, lack the sensitivity to detect low-abundance or emerging mutations reliably.
False negatives or missed mutations due to low sensitivity undermine clinical confidence in ESR1 testing, leading oncologists to hesitate in ordering these tests or relying on the results for treatment decisions. Inconsistent assay performance across labs creates variability and skepticism regarding test accuracy, slowing adoption and reimbursement.
The need for more sensitive, expensive technologies such as droplet digital PCR (ddPCR) or next-generation sequencing (NGS) limits accessibility, especially in resource-constrained settings. Without reliable detection, the clinical utility of ESR1 testing as a companion diagnostic for emerging SERDs is compromised, reducing pharma-driven market momentum.
Opportunities:
Companion diagnostics for next-gen SERDs create a market opportunity for the ESR1-mutated metastatic breast cancer diagnostics market
The development of next-generation selective estrogen receptor degraders (SERDs), such as elacestrant (Orserdu), has created a significant market opportunity for ESR1-mutated metastatic breast cancer diagnostics. These targeted therapies require precise identification of ESR1 mutations to determine patient eligibility, making companion diagnostics indispensable. Next-gen SERDs address resistance mechanisms mediated by ESR1 mutations that cause failure of traditional endocrine therapies. Accurate detection via companion diagnostics enables timely switching to effective therapies.
The approval of elacestrant by the FDA in January 2023 and the simultaneous approval of ESR1 mutation companion diagnostics have accelerated market adoption. This co-approval model boosts demand for reliable ESR1 testing. The rise of next-gen SERDs has tightly coupled the need for precise ESR1 mutation detection, positioning companion diagnostics as a critical market driver. This synergy between drug and diagnostic development fuels expansion in the ESR1-mutated metastatic breast cancer diagnostics market.
For more details on this report – Request for Sample
ESR1 Mutated Metastatic Breast Cancer Diagnostics Market, Segment Analysis
The global ESR1-mutated metastatic breast cancer diagnostics market is segmented based on test type, sample type, end-user, and region.
Next‑Generation Sequencing (NGS) based tests from the test type segment are expected to hold 67.39% of the market share in 2024 in the ESR1 mutated metastatic breast cancer diagnostics market
The NGS-based tests segment holds a dominant position in the ESR1 mutated metastatic breast cancer diagnostics market due to its high sensitivity, multiplexing capability, and comprehensive genomic profiling, which are essential for detecting complex ESR1 mutations and co-occurring resistance mutations. Clinical trials use NGS assays to identify ESR1 mutations, validating their role in real-world treatment pathways.
For instance, in March 2023, Sermonix Pharmaceuticals Inc. and Guardant Health, Inc. announced the initiation of a registrational Phase 3 clinical study comparing targeted lasofoxifene in combination with the CDK 4/6 inhibitor abemaciclib versus fulvestrant plus abemaciclib in pre- and post-menopausal subjects with locally advanced or metastatic ER+/HER2- breast cancer with an ESR1 mutation.
Next genome sequencing is also used in liquid biopsy samples, such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), or cell-free DNA (cfDNA). Liquid biopsies provide a minimally invasive method for monitoring the genomic changes in real-time, allowing for the detection of ESR1 mutations and other alterations.
ESR1 Mutated Metastatic Breast Cancer Diagnostics Market, Geographical Analysis
North America is expected to dominate the global ESR1 mutated metastatic breast cancer diagnostics market with a 41.32% share in 2024
The presence of state-of-the-art molecular diagnostic laboratories and market players such as Guardant Health developing diagnostics, which is further driving the market growth in the region. And widespread adoption of liquid biopsy technologies enables accurate, rapid ESR1 mutation detection. For instance, in January 2023, Guardant Health received FDA approval for Guardant360 CDx as a companion diagnostic for Menarini Group’s ORSERDU for the treatment of patients with ESR1 mutations in ER, HER2 advanced or metastatic breast cancer.
The U.S. market benefits from active partnerships between diagnostic companies and pharmaceutical firms developing ESR1-targeted therapies like elacestrant (approved by the FDA). The co-approval of companion diagnostics accelerates clinical adoption. Oncologists in the region are well-informed about the clinical relevance of ESR1 mutations, driven by ongoing research and participation in pivotal clinical trials (e.g., EMERALD, PADA-1). This awareness boosts diagnostic test utilization.
The combination of high disease burden, cutting-edge diagnostic infrastructure, strong pharma partnerships, regulatory support, and clinical awareness firmly establishes North America as the dominant region in the ESR1-mutated metastatic breast cancer diagnostics market.
Asia-Pacific is growing at the fastest pace in the ESR1 mutated metastatic breast cancer diagnostics market, holding 19.02% of the market share
The Asia-Pacific region is witnessing the fastest growth in the ESR1-mutated metastatic breast cancer diagnostics market due to rising breast cancer incidence, improving healthcare infrastructure, growing awareness, and increasing adoption of advanced diagnostic technologies. Countries like China, India, Japan, and South Korea report a significant increase in metastatic breast cancer cases, driving demand for advanced diagnostics like ESR1 mutation testing.
Regional diagnostic companies are collaborating with global leaders, such as Guardant Health, to expand ESR1 testing availability. Enhanced awareness campaigns and breast cancer screening programs across APAC are leading to early diagnosis and increased demand for precise mutation profiling to guide treatment.
ESR1 Mutated Metastatic Breast Cancer Diagnostics Market Competitive Landscape
Top companies in the ESR1 mutated metastatic breast cancer diagnostics market include Guardant Health, Inc., Illumina, Inc., APIS Assay Technologies, GENCURIX, Sysmex Corporation, ASURAGEN, INC., CUSABIO TECHNOLOGY LLC, and NeoGenomics Laboratories, among others.
ESR1 Mutated Metastatic Breast Cancer Diagnostics Market Scope
Metrics | Details | |
CAGR | 4.4% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Mn) | |
Segments Covered | Test Type | PCR‑Based Tests and Next‑Generation Sequencing (NGS)–Based Tests |
Sample Type | Plasma-Based and Tissue-Based | |
End-User | Hospitals, Diagnostic Centers, Oncology Centers, and Research & Academic Institutes | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global ESR1 mutated metastatic breast cancer diagnostics market report delivers a detailed analysis with 56 key tables, more than 49 visually impactful figures, and 146 pages of expert insights, providing a complete view of the market landscape.